07 February 2024: Articles 
Accelerating Healing and Relieving Pain: High-Intensity Laser Therapy for Paraneoplastic Cutaneous Vasculitis Associated with Multiple Myeloma
Unusual clinical course, Challenging differential diagnosis, Unusual setting of medical care, Educational Purpose (only if useful for a systematic review or synthesis)
Karina Felipe Fernandes Maciel1ABEF, Antonio Henrique Cordeiro2B, Rivadavio Fernandes Batista de Amorim1ACDEF, Ludmila Bertti Coelho2F*, Mariana de Freitas Cordeiro3ADE, Thiago David Alves Pinto1DOI: 10.12659/AJCR.942322
Am J Case Rep 2024; 25:e942322
Abstract
BACKGROUND: Leukocytoclastic vasculitis (LCV) is an atypical form of cutaneous paraneoplastic manifestation. Its association with multiple myeloma (MM) is even rarer and is associated with poor prognosis and short survival, regardless of the therapy instituted. Different treatment approaches are necessary. We present a case in which LCV was the first manifestation of MM, and high-intensity laser therapy (HILT) was used as an option to accelerate healing and control pain.
CASE REPORT: A 76-year-old woman presented with pain and paresthesia in her lower limbs, associated with palpable purpura. The clinical diagnosis was small-vessel vasculitis. Laboratory tests showed an elevated gamma globulin monoclonal peak on protein electrophoresis. The immunophenotypic study of bone marrow aspirates led to the diagnosis of MM. Due to pain refractory to conventional analgesics, and the progressive evolution of the lesions, despite corticosteroid therapy, we performed photo-biomodulation with a neodymium-doped yttrium aluminum garnet laser (Nd: YAG), wavelength 1064 nanometers, using a 7-mm probe and energy density 6 J/cm². After the first session, the patient was referred for pain management, and after 5 weeks, we observed complete healing in ulcerated lesions and involution of bullous lesions.
CONCLUSIONS: This case report shows the positive effects of the Nd: YAG laser in modulating healing and reducing pain. HILT is an innovative, non-invasive, and effective treatment and should be considered a promising technique to accelerate healing and controlling pain.
Keywords: Laser Therapy, Multiple Myeloma, Paraneoplastic Syndromes, Vasculitis, Wound Healing
Background
Cutaneous leukocytoclastic vasculitis (LCV) is a systemic inflammatory disorder involving the small vessels. It is characterized by segmental angiocentric neutrophilic inflammation, endothelial damage, and fibrinoid necrosis. LCV can clinically present in various forms, from urticarial macules to ulcerated and necrotic lesions [1,2]. Paraneoplastic vasculitis (induced by lymphoproliferative disease, myeloproliferative disease, or carcinoma) represents less than 5% of all cases of LCV [3]. The association with multiple myeloma (MM) is rare, and only 22 such cases have been reported in the literature [4].
Unfortunately, the management of paraneoplastic vasculitis is not standardized and has been associated with the treatment of underlying disease. Antihistamine drugs, non-steroidal anti-inflammatory drugs, corticosteroids, or immunosuppressants can be used [5,6].
Laser therapy has been used as an alternative to reduce pain and accelerate wound healing [7]. It is a non-invasive and painless physiotherapy modality consisting of low-level light therapy (LLLT) with energy output <500 mW, reaching only superficial tissues, and high-intensity laser therapy (HILT) with energy output >500 mW and can reach deeper tissues [7]. Some protocols with neodymium-doped yttrium aluminum garnet laser (Nd: YAG), wavelength 1064 nanometers, are considered HILT and have photo-biomodulation and anti-inflammatory effects [8].
This report is of a case in which cutaneous vasculitis had encouraged investigation for an underlying disease and determined the diagnosis of MM. HILT with Nd: YAG laser was performed to promote photo-biomodulation, accelerate inflammatory absorption, increase collagen synthesis and tensile strength, shorten wound healing time, reduce wound size [8], and control pain patterns [9].
Case Report
A 76-year-old woman presented with palpable purpura and painful ulcers 2 weeks before coming to our institution. The clinical signs were associated with asthenia, swelling, burning pain, dyskinesias, and paresthesia in the lower limbs 2 months before the cutaneous manifestation. She had no history of arterial or venous disease, only primary arterial hypertension. There was no history of previous neoplasia or similar clinical picture. On physical examination, the patient had palpable pulses in all limbs, and no varicose veins were detected. There was mild distal edema of the legs, palpable purpura, some coalescent, disseminated throughout the infragenicular region and feet, bilaterally (Figure 1). Two ulcerated lesions with irregular necrotic edges, approximately 1.5×2.0 cm and 0.5×0.5 cm in diameter, respectively, with inflammatory signs, were observed on the medial side of the ankle. Other scattered blistered and blackened lesions were also present. These lesions are uncommon in chronic venous insufficiency (CVI) and suggest vasculitis (Figure 2). The patient had no lymphadenopathy.
The diagnostic hypothesis was small-vessel vasculitis. Histopathological analysis of a skin lesion revealed vascular degeneration, mixed angiocentric infiltration containing lymphocytes, histiocytes, and neutrophils, mild red blood cell extravasation, and fibrinoid necrosis in vessel walls, indicating leukocytoclastic vasculitis (Figure 3).
Hence, additional tests were performed. The duplex scan eliminated CVI and peripheral arterial disease. Viral involvement or association with rheumatological disease was ruled out. Laboratory tests detected an increase in erythrocyte sedimentation rate and C-reactive protein, beta 2 microglobulin: 3.98 mcg/mL (reference range (RR): 1.09 to 2.53 mcg/mL) and a monoclonal gamma peak globulin in protein electrophoresis (2.402 g/dL, RR: absent). Serum IgG levels were slightly elevated at 3220.0 mg/dL (RR: 650 to 1600 mg/dL). Immunofixation of urinary proteins demonstrated the presence of a monoclonal IgG/Kappa component. Immunofixation electrophoresis detected a monoclonal IgG/kappa component and free light chain analysis was performed, showing a kappa chain level of 26.10 mg/L, a lambda chain level of 11.0 mg/L, and a kappa/lambda ratio of 2.373 (VR: 0.31 to 1.56), indicating kappa gammopathy (Table 1).
Culturing of a specimen showed a positive result for
The hematology team performed bone marrow puncture, which showed plasmacytosis on the myelogram. A bone marrow anatomopathological study showed hypercellularity due to atypical plasmocytosis (Figure 4A). The immunophenotypic assessment of bone marrow aspirate showed 0.32% of abnormal immunophenotypic plasmocytes expressing monoclonal kappa light chain (cytoplasmic). Immunohistochemical findings corroborated the diagnosis of MM CD138+ with kappa light chain expression (Figure 4B, 4C). Non-specific chemo-therapy was chosen because the MM stage did not fulfill the criteria for transplantation or chemotherapy.
Due to pain refractory to conventional analgesics and the progressive evolution of lesions despite oral corticosteroid therapy, HILT photo-biomodulation was initiated. The parameters used were: 1064 nanometers wavelength laser, micro-pulses of 300 microseconds duration, a 7-mm probe, and energy density 6 J/cm2. The exposure time and amount of energy supplied were calculated by the formula: Power=Energy/Time. A thermography camera was used as a safety measure to avoid application errors or burns. Laser therapy sessions were performed twice a week. A healing rate of 0.53, approximately 7 mm2 per day, was achieved in ulcerated lesions and involution of bullous lesions after 10 sessions in 5 weeks.
There was considerable improvement in the pain pattern and edema of the lower limbs after control of cutaneous vasculitis. The skin still presents post-inflammatory hyperchromic macules without atrophic or dystrophic scars (Figure 5). After the lesions healed, the dose of prednisone was gradually reduced until complete suspension. Eight months later, new lesions appeared, and the same treatment was successfully instituted. Currently, the treatment with corticosteroids continues, with a daily oral dose of 5 mg of prednisone, and the patient remains asymptomatic in hematological follow-up without chemotherapy treatment or bone marrow transplant.
Discussion
The association between LCV and MM is rarely observed. LCV is related to lymphoproliferative diseases in only 1% of cases [3,4]. Its development has been linked to cryoglobulinemia, infections, and hypersensitivity to drugs, and rarely presents as paraneoplastic syndrome [11].
The possible mechanisms responsible for developing vasculitis include abnormal production of immunoglobulins in response to abnormal tumor cells and vascular antigens of the endothelium, forming either in situ immune complexes or circulating immune complexes that deposit on the vessel walls. Impaired clearance of typically produced immune complexes is also postulated [11,12].
LCV may manifest before the diagnosis of malignancy, be concomitant, or indicate a recurrence. In some patients, it precedes the emergence of clinical manifestations of neoplasia by 2–4 years [2,4].
Only 22 cases associating MM and vasculitis have been reported [4,13–24]. In a review conducted at the Myeloma Institute of the University of Arkansas between January 1989 and July 2001, 8 cases of LCV associated with MM were found among 2357 patients. LCV may be diagnosed simultaneously with MM or preceded other signs and symptoms of multiple myeloma by a few years. In this case, it preceded MM by several weeks. The predominance of IgG paraprotein was observed in 60% of cases and IgA in 20–25% [4]. The reported case is also related to the monoclonal component of IgG/kappa, as in most of the 22 reported cases in the literature [2,4,11,14–25] (Table 2). In addition, the prognosis was favorable, and lesions healed after the institution of appropriate treatment: chemotherapy in 10 cases and 1 case after bone marrow transplant [2,4,11,14–25]. Two patients died before recovering, and there were no references in 9 cases [4,14,20].
Therefore, the prognosis of vasculitis relies on the evolution and treatment of the underlying disease [5,6]. In the reported case, non-specific chemotherapy was chosen because the patient did not have anemia, hyperkalemia, bone lesions, kidney damage, or hyperviscosity and did not fulfill the criteria for transplantation or chemotherapy. Oral corticosteroids alone were not effective in healing the skin lesions. Therefore, HILT was an alternative to accelerate the tissue healing process, control pain, and assist the action of corticosteroid therapy. The Brazilian Society of Dermatology has published the Brazilian consensus on the diagnosis and management of chronic leg ulcers in 2020 and considers laser therapy to be a promising treatment [26].
The primary biological effects of laser therapy on biological systems include mechanical, electrical, thermal, photochemical, and bio-stimulating effects [27]. Laser therapy with the wavelength of 1064 nm provides the best penetration into tissues, and its absorption by some tissue chromophores (such as hemoglobin, melanin, and water) is lower, thereby avoiding creation of thermal lesions and enhancing the wound-healing process [27–29].
A cascade of metabolic effects results in several physiological changes, causing better tissue repair, faster resolution of the inflammatory response, and reduced pain [30]. The 1064-nm laser wavelength delivers a specific dose of energy to the areas of the tissue to be treated, providing photo-biomodulation at the cellular level by inducing endogenous ATP synthesis and increasing oxygen consumption, the activity of membrane enzymes, and the synthesis of DNA and RNA [31,32]. At the tissue level, there is a decrease in inflammatory mediators by the activation of lymphocytes and macrophages secretion of fibro-blast growth factors, in addition to fibrin reabsorption, collagen remodeling, neoangiogenesis, and vasodilation [28,29,33].
Some reports have found HILT to have anti-inflammatory and analgesic effects in patients with musculoskeletal disorders by slowing the transmission of pain stimuli and increasing the production of morphine-mimetic substances [9,34]. In the literature, the analgesic effect of HILT is described as immediate and long-lasting, as it was in our case [35].
Notably, some studies have demonstrated the effectiveness of HILT in accelerating wound healing [8,27,28,30,31,34,36]. Nd: YAG laser irradiation effectively inhibits the growth of
An experimental study by Mahran compared the effects of high- and low-intensity lasers on the healing of acute wounds induced on the back of diabetic rats. Histological analysis indicated that epithelialization was faster in those undergoing laser therapy vs the control group and that there was no difference between the groups treated with LLLT and HILT [28].
In 2018, Alayat’s meta-analysis included 122 patients. It concluded that a high-intensity laser is a safe and effective alternative in the healing of neuropathic ulcers, reducing the size of lesions and reducing their closing time [30].
As in prior studies, the present case demonstrated that HILT accelerated wound healing time and reduced pain patterns.
Conclusions
This case demonstrates that leukocytoclastic vasculitis can precede other signs and symptoms of multiple myeloma and should alert clinicians to the possibility of this diagnosis. HILT can be a painless, safe, and effective alternative treatment of vasculitis. The disadvantages of HILT include the cost and the need for technical knowledge to prevent undesirable effects such as burns. Nonetheless, further cost-benefit studies are necessary to validate HILT protocols with Nd: YAG for these purposes.
Figures
References:
1.. Carlson JA, Chen KR, Cutaneous vasculitis update: Small vessel neutrophilic vasculitis syndromes: Am J Dermatopathol, 2006; 28(6); 486-506
2.. Kurzrock R, Cohen PR, Vasculitis and cancer: Clin Dermatol, 1993; 11(1); 175-87
3.. Loricera J, Calvo-Río V, Ortiz-Sanjuán F, The spectrum of paraneoplastic cutaneous vasculitis in a defined population: incidence and clinical features: Medicine (Baltimore), 2013; 92(6); 331-43
4.. Bayer-Garner IB, Smoller BR, Leukocytoclastic (small vessel) vasculitis in multiple myeloma: Clin Exp Dermatol, 2003; 28(5); 521-24
5.. Brandt HRC, Arnone M, Valente NYS, [Cutaneous small vessel vasculitis: Subtypes and treatment – Part II.]: An Bras Dermatol, 2007; 82(6); 499-511 [in Portuguese]
6.. Kermani TA, Warrington KJ, Dua AB, Treatment guidelines in vasculitis: Rheum Dis Clin North Am, 2022; 48(3); 705-24
7.. Karu TI, Kolyakov SF, Exact action spectra for cellular responses relevant to phototherapy: Photomed Laser Surg, 2005; 23(4); 355-61
8.. Lu Q, Yin Z, Shen X, Clinical effects of high-intensity laser therapy on patients with chronic refractory wounds: A randomised controlled trial: BMJ Open, 2021; 11(7); e045866
9.. Arroyo-Fernández R, Aceituno-Gómez J, Serrano-Muñoz D, Avendaño-Coy J, High-intensity laser therapy for musculoskeletal disorders: A systematic review and meta-analysis of randomized clinical trials: J Clin Med, 2023; 12(4); 1479
10.. Finnie A, The SIGN guideline on the care of chronic leg ulcers: an aid to improving practice: J Wound Care, 2000; 9(8); 365-67
11.. Bayer-Garner IB, Smoller BR, The spectrum of cutaneous disease in multiple myeloma: J Am Acad Dermatol, 2003; 48(4); 497-507
12.. Buggiani G, Krysenka A, Grazzini M, Paraneoplastic vasculitis and paraneoplastic vascular syndromes: Dermatol Ther, 2010; 23(6); 597-605
13.. Fortin PR, Vasculitides associated with malignancy: Curr Opin Rheumatol, 1996; 8(1); 30-33
14.. Kembre PS, Mahajan S, Kharkar V, Khopkar U, Cutaneous vasculitis as a presenting feature of multiple myeloma: A report of 2 cases: Indian J Dermatol Venereol Leprol, 2006; 72(6); 437-39
15.. Highet AS, Urticarial vasculitis and IgA myeloma: Br J Dermatol, 1980; 102(3); 355-57
16.. Raper RF, Ibels LS, Osteosclerotic myeloma complicated by diffuse arteritis, vascular calcification and extensive cutaneous necrosis: Nephron, 1985; 39(4); 389-92
17.. McMillen JJ, Krueger SK, Dyer GA, Leukocytoclastic vasculitis in association with immunoglobulin A myeloma: Ann Intern Med, 1986; 105(5); 709-10
18.. Oymanns M, Baltaci M, Bellm A, Assaf C, Paraneoplastic filiform hyperkeratosis and immunoglobulin-associated vasculitis in myeloma progression: A case report: Case Rep Dermatol, 2021; 13(3); 563-67
19.. Oka S, Ono K, Nohgawa M, Multiple myeloma presenting as cutaneous leukocytoclastic vasculitis and eosinophilia disclosing a T helper type 1/T helper type 2 imbalance: A case report: J Med Case Rep, 2018; 12(1); 320
20.. Marini A, Fenk R, Plettenberg H, [Rare types of vasculitis as markers of plasmocytoma]: Hautarzt, 2006; 57(2); 137-43 [in German]
21.. Sánchez NB, Canedo IF, García-Patos PE, Paraneoplastic vasculitis associated with multiple myeloma: J Eur Acad Dermatol Venereol, 2004; 18(6); 731-35
22.. Abouzaid C, Zahlane M, Benjilali L, [Paraneoplastic cutaneous leukocytoclastic vasculitis disclosing IgA multiple myeloma]: Presse Med, 2013; 42(4 Pt 1); 482-84 [in French]
23.. Jain P, Kumar P, Parikh PM, Multiple myeloma with paraneoplastic leucocytoclastic vasculitis: Indian J Cancer, 2009; 46(2); 173-74
24.. Lamaison DB, Silva IL, Magro ACD, [Leukocytoclastic vasculitis secondary to multiple myeloma.]: Hematol Transfus Cell Ther, 2020; 42(Suppl. 2); 281 [in Portuguese]
25.. Akpunonu B, Sabgir D, Panchal K, Kahaleh B, An elderly man with vasculitis and IgA myeloma: J Eur Acad Dermatol Venereol, 1998; 10(2); 186-87
26.. Abbade LPF, Frade MAC, Pegas JRP, Consensus on the diagnosis and management of chronic leg ulcers – Brazilian Society of Dermatology: An Bras Dermatol, 2020; 95(Suppl. 1); 1-18
27.. da Silva JP, da Silva MA, Almeida AP, Laser therapy in the tissue repair process: A literature review: Photomed Laser Surg, 2010; 28(1); 17-21
28.. Mahran HG, Faruk EM, ElSawy NA, Alkushi AG, Pulsed high-intensity Laser versus Low-intensity laser on healing of full-thickness wound in diabetic rats. (Histological and immunohistochemical study): International Journal of Pharma and Bio Sciences, 2017; 8(2); 874-88
29.. Hong SE, Hong MK, Kang SR, Young Park B, Effects of neodymium-yttrium-aluminum garnet (Nd: YAG) pulsed high-intensity laser therapy on full thickness wound healing in an experimental animal model: J Cosmet Laser Ther, 2016; 18(8); 432-37
30.. Alayat MS, El-Sodany AM, Ebid AA, Efficacy of high intensity laser therapy in the management of foot ulcers: A systematic review: J Phys Ther Sci, 2018; 30(10); 1341-45
31.. Amaroli A, Benedicenti A, Ravera S, YAG 1064nm) laser irradiation photobiomodulates mitochondria activity and cellular multiplication of Paramecium primaurelia (Protozoa): Eur J Protistol, 2017; 61(Pt A); 294-304
32.. Tkocz P, Matusz T, Kosowski Ł, A randomised-controlled clinical study examining the effect of high-intensity laser therapy (HILT) on the management of painful calcaneal spur with plantar fasciitis: J Clin Med, 2021; 10(21); 4891
33.. Bostanciklioglu M, Demiryürek Ş, Cengiz B, Assessment of the effect of laser irradiations at different wavelengths (660, 810, 980, and 1064 nm) on autophagy in a rat model of mucositis: Lasers Med Sci, 2015; 30(4); 1289-95
34.. Alayat MSM, Aly THA, Elsayed AEM, Aly THA, Elsayed AEM, Fadil ASM, Efficacy of pulsed Nd: YAG laser in the treatment of patients with knee osteoarthritis: A randomized controlled trial: Lasers Med Sci, 2017; 32(3); 503-11 [Erratum in: Lasers Med Sci. 2020;35(8):1875]
35.. Szabo DA, Neagu N, Teodorescu S, TECAR therapy associated with high-intensity laser therapy (HILT) and manual therapy in the treatment of muscle disorders: A literature review on the theorised effects supporting their use: J Clin Med, 2022; 11(20); 6149
36.. Ebid AA, Alhammad RM, Alhindi RT: J Phys Ther Sci, 2021; 33(3); 222-28
Figures
Tables
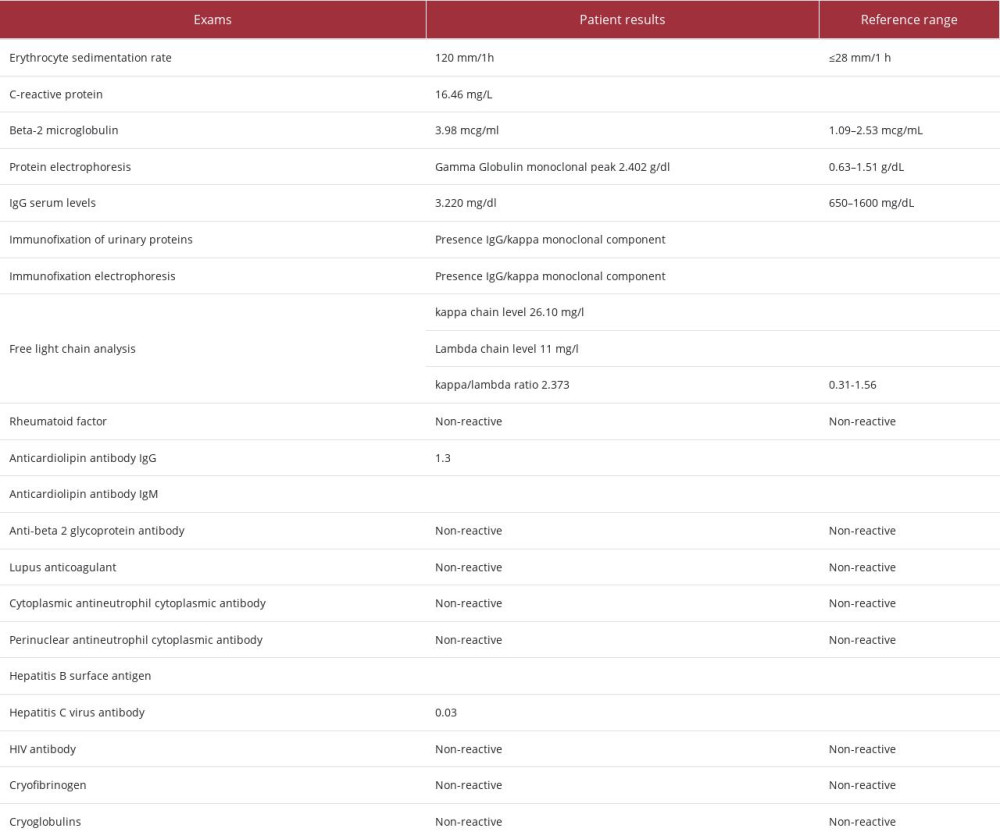 Table 1.. Laboratory exams of the patient.
Table 1.. Laboratory exams of the patient.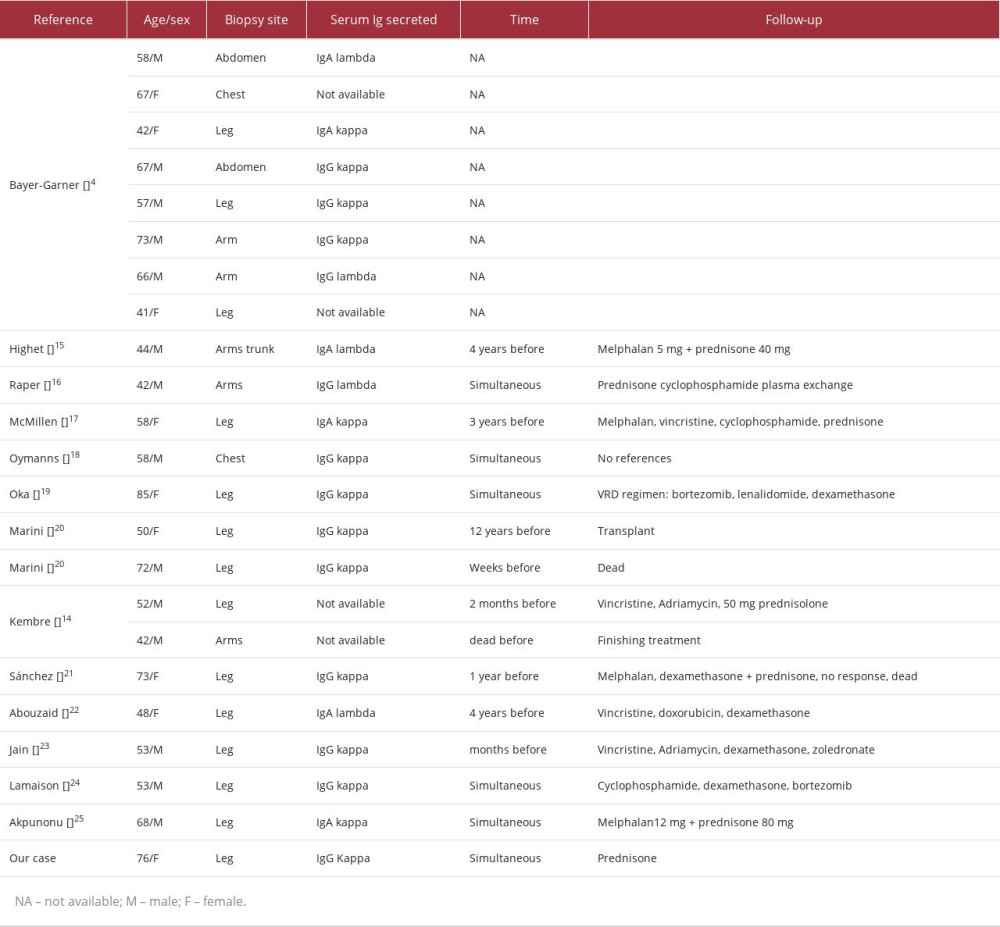 Table 2.. Features of the 22 patients with coexistent multiple myeloma and leukocytoclastic vasculitis and the related case.
Table 2.. Features of the 22 patients with coexistent multiple myeloma and leukocytoclastic vasculitis and the related case. Table 1.. Laboratory exams of the patient.
Table 1.. Laboratory exams of the patient. Table 2.. Features of the 22 patients with coexistent multiple myeloma and leukocytoclastic vasculitis and the related case.
Table 2.. Features of the 22 patients with coexistent multiple myeloma and leukocytoclastic vasculitis and the related case. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








