25 January 2024: Articles 
Lymphoscintigraphy and Single-Photon Emission Computed Tomography (SPECT)/CT to Determine Need for Second Sentinel Lymph Node Biopsy for Breast Cancer Recurrence Following Ipsilateral Breast/Axillary Surgery
Unusual clinical course, Challenging differential diagnosis
Satoko NakanoDOI: 10.12659/AJCR.942424
Am J Case Rep 2024; 25:e942424
Abstract
BACKGROUND: For patients with cN0 breast cancer, sentinel node biopsy (SNB) is performed to confirm metastasis. When cancer recurs after a breast/axillary surgery, performing a second SNB is debatable in terms of its accuracy and significance. However, SNB is often performed because it is less invasive and can provide significant information. This report describes our experience of performing lymphoscintigraphy and single-photon emission computed tomography (SPECT)/CT to determine whether SNB is informative or not in patients who develop ipsilateral breast tumor recurrence (IBTR) following a breast/axillary surgery.
CASE REPORT: We included 9 patients with breast cancer and a history of ipsilateral breast/axillary surgery who underwent lymphoscintigraphy and SPECT/CT between April 2020 and July 2023. For lymphoscintigraphy, 20-25 MBq of 99mTc-phytate was injected subcutaneously in the areola, and planar images and SPECT/CT were taken at 15 min and 3 h after the injection. In lymphoscintigraphy, radioisotope accumulation was detected in 2 patients at 15 min and 8 patients at 3 h; it was not detected in 1 patient. The accumulation site was only the axilla in 3 patients; other sites including the axilla in 3, and sites outside the axilla in 2.
CONCLUSIONS: When a patient who previously underwent breast/axillary surgery develops IBTR, the initial surgery may have altered the lymphatic flow. The lymphatic flow varied between the contralateral or ipsilateral internal mammary lymph nodes, contralateral axilla, multidirectional flow, and the axilla alone. Lymphoscintigraphy and SPECT/CT may be useful for early determination of the need for another SNB.
Keywords: lymphoscintigraphy, Neoplasm Recurrence, Local, Sentinel Lymph Node Biopsy, Tomography, Emission-Computed, Single-Photon
Background
Sentinel node biopsy (SNB) has become the standard of care for patients with cN0 breast cancer, and long-term results have been obtained [1–8]. However, evidence to support the use of second SNB in patients with ipsilateral breast tumor recurrence (IBTR) following an ipsilateral breast or axillary surgery remains insufficient. The less invasive nature of second SNB is a major advantage and has no reported definite disadvantage; thus, second SNB is performed in clinical practice. The lymphatic flow is assumed to be altered by the initial surgery; hence, many lymphoscintigraphy studies have been conducted to confirm this assumption. Performing second SNB is reasonable after verifying the location of the sentinel node (SN) in advance. In this report, we describe our experience of performing lymphoscintigraphy and single-photon emission computed tomography (SPECT)/CT in advance to determine whether SNB should be performed in patients who previously underwent ipsilateral breast or axillary surgery.
Case Report
This study included 9 patients with breast cancer and a history of ipsilateral breast or axillary surgery who underwent lymphoscintigraphy and SPECT/CT between April 2020 and July 2023. Lymphoscintigraphy was performed on a different day than surgery. Planar and SPECT/CT images were taken at 15 min and 3 h after 20–25 MBq of 99mTc-phytate was injected subcutaneously into the nipple area. SNB was performed during surgery using 2 mapping techniques: with isotope and with indigo carmine.
Patients provided written consent after being informed of the purpose of the exploratory study. Data were collected retrospectively from electronic medical records, and the institutional review board approved this study.
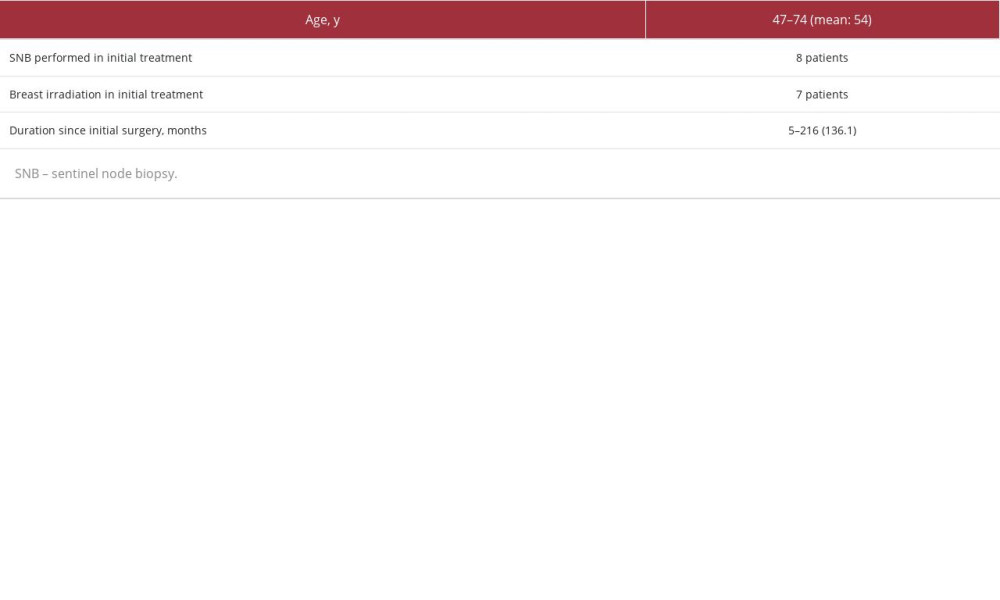
All 9 patients aged 47–74 years (mean: 54 years) were clinically node-negative and had no metastasis. The duration after the initial surgery was 5 months to 18 years (mean: 136 months) (Table 1). Lymphoscintigraphy was performed before the surgery.
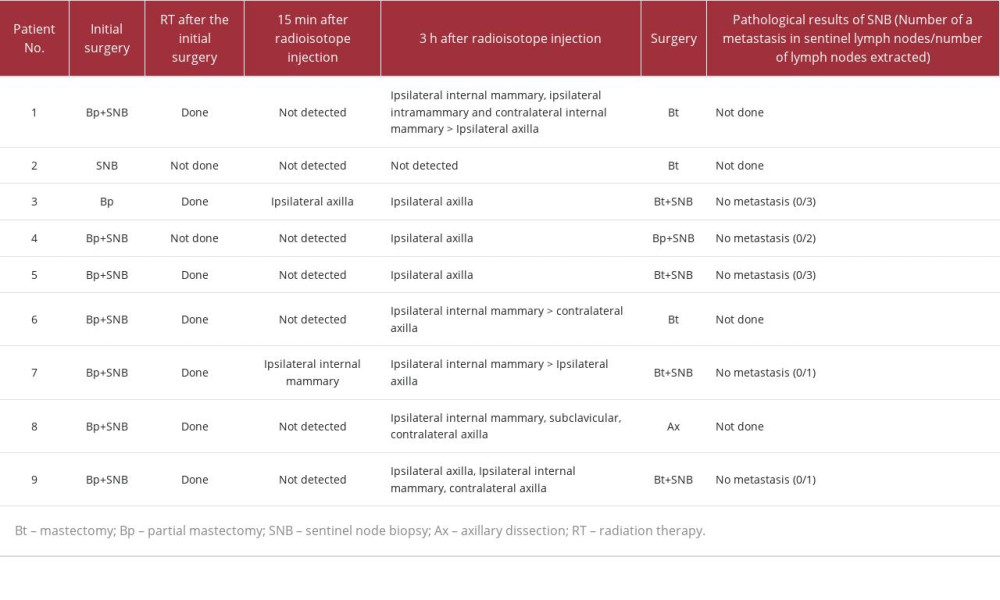
As shown in Table 2, radioisotope accumulation by lymphoscintigraphy was observed in 2 patients at 15 min and 8 patients at 3 h, while SN could not be detected in 1 patient. The accumulation sites were only the axilla in 3 patients, other sites including axilla in 3, and sites other than the axilla in 2. Intraoperative SNB was performed in 5 of the 6 patients with lymphatic flow to the axilla (Patients 3, 4, 5, 7, and 9). Although the remaining patient also had lymphatic flow to the axilla, intraoperative SNB was not performed because the nonaxillary lymph nodes were dominant
Figure 1 shows the cases subjected to undergo second SNB according to the lymphoscintigraphic findings. Patient 1, who underwent breast-conserving surgery (BCS) plus SNB as an initial surgery 18 years ago, received mastectomy without second SNB according to lymphoscintigraphy, which detected ipsilateral and contralateral internal mammary lymph node (LN) dominantly and ipsilateral axillar LN at 3 h after 99mTc-phy-tate injection. A second SNB was not performed during the surgery because the axillary lymph nodes were not dominant.
Patient 2 had triple-negative breast cancer that was clinically node-negative. She had SNB initially and then neoadjuvant chemotherapy. However, the cancer progressed. Thus, the treatment was changed to surgery. Lymphoscintigraphy and SPECT/ CT were performed at first and showed no radioisotope accumulation at 15 min and at 3 h. Thus, only mastectomy was performed (Figure 2).
Patient 4 (Figure 3) underwent BCS plus SNB 14.5 years earlier. Although radiotherapy (RT) to the affected breast was recommended, consent was not obtained; thus, RT was not performed after the initial surgery. The lymphoscintigraphy and SPECT/CT showed radioisotope accumulation only in the axilla at 15 min and at 3 h. Hence, BCS plus a second SNB was performed, followed by RT to the breast.
Surgical methods were determined according to the dominant site of radioisotope accumulation for each case.
However, second SNB was not performed in 1 patient because no LN was detected, and in 3 patients because the lymphatic flow went only to the outside of the axilla.
Discussion
SNB emerged in the mid-1990s and was initially reported in many cases using lymphoscintigraphy mapping of the lymphatic flow from the breast [3,4,7,8]. Jansen et al reported that 79% of patients had SN only in level I or II axillae, 16% in axillae and nonaxillae, and only 3% in non-axillae [8]. Although SN is not noted in axillary level I and II and is sometimes detected in LNs above level III or outside of the axilla [3,6–8], gamma scintillation counters have shown a high intraoperative detection rate (over 94%) [1,2,4]. The accuracy, sensitivity, and specificity of this tool are reportedly 96% or higher, 90% or higher, and 100%, respectively. However, SNB itself is informative and less invasive; hence, it has been a standard procedure for cN0 breast cancer [1–5].
The number of long-term follow-up cases after partial mastectomy and SNB has increased, and IBTR, which is related to a new primary breast cancer and the recurrence of a positive margin (mainly ductal component), has also become prevalent [9–11]. According to the National Comprehensive Cancer Network guidelines, the consensus recommendation of the panel for most patients with local recurrence following breast-conserving therapy and sentinel LN biopsy is mastectomy and a level I/II axillary dissection, given the lack of sufficient evidence regarding detection rate, false-negative rate, and prognosis [12].
The initial surgery may have affected the lymphatic flow. In this case, the requirement of a second SNB is debatable. Regarding adverse events, SNB is less invasive than axillary lymph node dissection (ALND). In addition, second SNB allows for the reevaluation of the disease stage. Several studies have reported detection through second SNB after initial surgery [13–18].
Generally, the detection rate of second SNB is 50-65%, and mapping by lymphoscintigraphy identifies SNs at a higher rate than intraoperative search using a gamma scintillation counter. The detection rate is also higher in SNB than in ALND in the initial treatment, and without radiation therapy (RT) than that with RT. Furthermore, the regional LN recurrence rate after second sentinel LN biopsy is reported to be less than 5% [15,19].
When a second SNB is performed intraoperatively with a gamma scintillation counter, 3 patterns are possible: (1) a SN is detected only in the axilla, (2) a SN is detected outside of the axilla, or (3) no SN is detected. If a SN is detected in the axilla, SNB would be performed as usual. If a SN is detected outside of the axilla, it is often impossible to detect intraoperatively. The gamma scintillation counter in the operating room does not provide a large field of view, so the search area is limited to the axilla and internal mammary lesion. In the present study, LNs were detected, particularly the ipsilateral, and contralateral internal mammary nodes and contralateral axillary nodes. Of note, LNs outside of the axilla are not included in the scope of local treatment, and biopsy, and pathological examination of these areas would be overly invasive. Dissection is always invasive, and it would not prove the absence of metastases. If the second SN is in a different region without LN metastases, dissection is a pointless over-invasive procedure.
In clinical practice, many possible situations are expected, but SNB itself is minimally invasive; if second SN is detected in the axilla, SNB may be performed. However, it is uncertain whether the results are as accurate and prognostic as the initial SNB. This uncertainty is most likely caused by the lack of knowledge of the lymphatic flow; mapping by lymphoscintigraphy will eliminate this uncertainty.
In the present study, lymphoscintigraphy was performed to clarify this point; planar images were taken, followed by SPECT/CT, which made the LN location easily identified [9,20]. This advantage is a sufficient reason to perform second SNB for breast cancer recurrence with a history of breast or axillary surgery.
Second SNB detected no metastases in all cases. According to some reports, when SNs can be accurately assessed, appropriate adjuvant therapy can be provided [21,22].
The use of lymphoscintigraphy to determine the indication for second SNB has been widely reported [9–18]. In particular, the use of SPECT/CT helps identify the SN location and increases the detection rate [10,20], supporting the present study. We did not observe metastasis in patients who underwent second SNB. The SNARB study reported that axillary lymph node recurrence was 1.0% after a median follow-up of 4.7 years without metastases after a second SNB for IBTR [19].
However, if the SN exhibits metastasis, applying the results of the American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011) trial, which showed no difference in 10-year survival between omitting and adding axillary dissection when only a few metastases were detected after sentinel node biopsy in patients with invasive breast cancer (cT1-2 and cN0), to the second SNB is debatable [23] and requires further confirmatory studies. Although the newer methods of SNB, such as ICG, SPIO, and CEUS, have been reported and compared with conventional methods [24–26], there are no reports available on their use in a second SNB. In the case of IBTR, conducting a study under uniform conditions remains difficult, given the wide variations in the location and size of the wound and postoperative treatment, such as radiotherapy and adjuvant therapy. In addition, the indications for SNB have been expanded due to fewer disadvantages. In particular, clinically node-positive cases with targeted axillary dissection/tailored axillary surgery are now being considered. Lymphatic flow might have been altered during clinical node positivity. The accuracy of the second SNB after the modification of neoadjuvant chemotherapy is uncertain.
In terms of information, lymphoscintigraphy seems to be the most informative considering that adding SPECT/CT to planar imaging provides current information on lymphatic flow and allows us to locate the SN. Although LNs outside the axilla were detected by lymphoscintigraphy and were not pathologically examined, possible systemic treatment, and RT may be considered depending on the status of the primary tumor and SNs. In addition, the SNs as the first lymphatic basin should be considered even though the LNs are anatomically distant; their progress should also be monitored.
This study has some limitations. This single-center study included a small number of patients. Furthermore, all patients who underwent a second SNB were node-negative. Long-term follow-up is required.
Conclusions
SNB is less invasive, and information can be obtained if a second SN can be detected. On the other hand, the initial surgery may have altered lymphatic flow, so it is unclear if only the axilla is examined intraoperatively. There need to be sufficient data on long-term axillary recurrence or overall survival rate. Although the situation varies in each patient, previous lymphoscintigraphy and SPECT/CT can provide information on altered lymphatic flow, which can evaluate the significance of performing a second SN and affect the choice of treatment options.
Prior lymphoscintigraphy and SPECT/CT are strongly recommended if there is a history of ipsilateral breast/axillary surgery.
Figures
References:
1.. Krag D, Weaver D, Ashikaga T, The sentinel node in breast cancer a multicenter validation study: N Engl J Med, 1998; 339; 941-46
2.. Veronesi U, Paganelli G, Viale G, A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer: N Engl J Med, 2003; 349; 546-53
3.. Giuliano AE, Kirgan DM, Guenther JM, Morton DL, Lymphatic mapping and sentinel lymphadenectomy for breast cancer: Ann Surg, 1994; 220; 391-98
4.. Cox CE, Pendas S, Cox JM, Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer: Ann Surg, 1998; 227; 645-51
5.. Lyman GH, American Society of Clinical Oncology guideline recommendation for sentinel lymph node biopsy in early-breast cancer: J Clin Oncol, 2005; 23; 7703-20
6.. van der Ploeg IM, Tanis PJ, Valdés Olmos RA, Breast cancer patients with extra-axillary sentinel nodes only may be spared axillary lymph node dissection: Ann Surg Oncol, 2008; 15; 3239-43
7.. Harlow S, Krag D, Weaver D, Ashikaga T, Extra-axillary sentinel lymph nodes in breast cancer: Breast Cancer, 1999; 6; 159-65
8.. Jansen L, Doting MH, Rutgers EJ, de Vries J, Clinical relevance of sentinel lymph nodes outside the axilla in patients with breast cancer: Br J Surg, 2000; 87; 920-25
9.. Taback B, Nguyen P, Hansen N, Sentinel lymph node biopsy for local recurrence of breast cancer after breast-conserving therapy: Ann Surg Oncol, 2006; 13; 1099-104
10.. Ge I, Erbes T, Juhasz-Böss I, Prognostic value and management of regional lymph nodes in locoregional breast cancer recurrence: A systematic review of the literature: Arch Gynecol Obstet, 2022; 306; 943-57
11.. Uth CC, Christensen MH, Oldenbourg MH, Sentinel lymph node dissection in locally recurrent breast cancer: Ann Surg Oncol, 2015; 22; 2526-31
12.. [cited 2023 Sep 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
13.. Karanlik H, Ozgur I, Kilic B, Sentinel lymph node biopsy and aberrant lymphatic drainage in recurrent breast cancer: Findings likely to change treatment decisions: J Surg Oncol, 2016; 114; 796-802
14.. Intra M, Trifirò G, Viale G, Second biopsy of axillary sentinel lymph node for reappearing breast cancer after previous sentinel lymph node biopsy: Ann Surg Oncol, 2005; 12; 895-99
15.. Intra M, Viale G, Vila J, Second axillary sentinel lymph node biopsy for breast tumor recurrence: Experience of the European Institute of Oncology: Ann Surg Oncol, 2015; 22; 2372-77
16.. Tokmak H, Kaban K, Muslumanoglu M, Management of sentinel node re-mapping in patients who have second or recurrent breast cancer and had previous axillary procedure: World J Surg Oncol, 2014; 12; 205
17.. Savolt A, Cserni G, Lazar G, Sentinel lymph node biopsy following previous axillary surgery inrecurrent breast cancer: Eur J Surg Oncol, 2019; 45; 1835-38
18.. Dinan D, Nagle CE, Pettinga J, Lymphatic mapping and sentinel node biopsy in women with an ipsilateral second breast carcinoma and a history of breast and axillary surgery: Am J Surg, 2005; 190; 614-17
19.. Poodt IGM, Vugts G, Maaskant-Braat AJG, Risk of regional recurrence after negative repeat sentinel lymph node biopsy in patients with ipsilateral breast tumor recurrence: Ann Surg Oncol, 2018; 25; 1312-21
20.. Borrelli P, Donswijk ML, Stokkel MP, Contribution of SPECT/CT for sentinel node localization in patients with ipsilateral breast cancer relapse: Eur J Nucl Med Mol Imaging, 2017; 44; 630-37
21.. Maaskant-Braat AJG, Roumen RMH, Voogd AC, Sentinel node and recurrent breast cancer (SNARB): Results of a nationwide registration study: Ann Surg Oncol, 2013; 20; 620-26
22.. Cordoba O, Perez-Ceresuela F, Espinosa-Bravo M, Detection of sentinel lymph node in breast cancer recurrence may change adjuvant treatment decision in patients with breast cancer recurrence and previous axillary surgery: Breast, 2014; 23; 460-65
23.. Giuliano AE, Ballman K, Mccall L, MS , Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: Long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial: Ann Surg, 2016; 264; 413-20
24.. Mathelin C, Lodi M, Narrative review of sentinel lymph node biopsy in breast cancer: A technique in constant evolution with still numerous unresolved questions: Chin Clin Oncol, 2021; 10; 20
25.. Ferrucci M, Franceschini G, Douek M, New techniques for sentinel node biopsy in breast cancer: Transl Cancer Res, 2018; 7(Suppl. 3); S405-17
26.. Rocco N, Velotti N, Pontillo M, New techniques versus standard mapping for sentinel lymph node biopsy in breast cancer: A systematic review and meta-analysis: Updates Surg, 2023; 75; 1699-710
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250