08 January 2024: Articles 
A 65-Year-Old Man Presenting to the Emergency Department with Gastric Hemorrhage Caused by a Glomus Tumor
Challenging differential diagnosis, Rare disease
Mariana Deacu12ABG, Madalina Bosoteanu12CD, Cristian-Ionut OrășanuDOI: 10.12659/AJCR.942610
Am J Case Rep 2024; 25:e942610
Abstract
BACKGROUND: Glomus tumor is a benign but rapidly growing mesenchymal tumor that is a rare in the gastrointestinal tract, can be locally invasive due to its rapid growth, and can result in perforation of a viscus. We report a 65-year-old man presenting as an emergency with gastric hemorrhage and gastric glomus tumor.
CASE REPORT: A 65-year-old man came to our hospital for a life-threatening upper digestive hemorrhage. The preoperative examinations (digestive endoscopy without sampling of biopsy fragments and contrast-enhanced computer tomography) led to the presumptive diagnosis of gastrointestinal stromal tumor. Wedge resection of the gastric wall was performed. The histopathological examinations revealed a proliferation of round-oval cells of medium size with a solid disposition and in nests. This proliferation dissected the muscular tunic and caused ulceration of the gastric mucosa. Immunohistochemical tests confirmed the diagnosis of glomus tumor and excluded other diagnoses (neuroendocrine tumor or gastrointestinal stromal tumor). The postoperative evolution was favorable, and at the time of discharge, the biochemical test values normalized.
CONCLUSIONS: Pathologists are faced with a challenging task due to the deceptive appearance that can be presented by such a rare tumor. Histopathological and immunohistochemical examinations are essential in achieving a precise diagnosis and assessing the biological potential of the glomus tumor. Even if it is a benign tumor, the clinical picture it causes can still be a major risk to the patient’s life. Consequently, ensuring effective case management becomes crucial, as it requires a thorough comprehension of all conditions encompassed in the differential diagnosis.
Keywords: Gastrointestinal Hemorrhage, gastrointestinal stromal tumors, Glomus Tumor, Immunohistochemistry
Background
Glomus tumors are rare mesenchymal proliferations, frequently located at the distal level of the extremities [1]. Glomus tumors represent about 1.5% of soft tissue tumors and can take 2 forms: sporadic, which are usually solitary lesions, or familial, which are usually multifocal and found in children [2].
The glomus apparatus is an arterio-venous system with a role in regulating body temperature. It consists of an afferent arterial vessel, efferent venous system, and convoluted channel. The canal is lined with a single row of epithelioid cells, namely glomus cells [3]. Glomus cells are modified smooth muscle cells that are small, round, and uniform with well-defined edges, centrally arranged nuclei, and indistinct nucleoli. The elements common to smooth muscle are found in the markers expressed by the glomus cell (caldesmon, smooth muscle actin, calponin), but the difference is the increased production of collagen IV and the absence of desmin [4].
Localization of these tumors at the gastric level is very rare, representing less than 1% of gastrointestinal soft tissue tumors. So far, a little over 200 cases have been described in the specialized literature [5]. At this level, glomus tumors are part of the category of subepithelial lesions of the gastrointestinal tract and are often confused with other more common entities, such as gastrointestinal stromal tumors, neuroendocrine tumors, or lymphomas [6].
The aim of this study is to report a case of a 65-year-old man with multiple cardiovascular diseases who presented as an emergency with gastric hemorrhage and received a diagnosis of gastric glomus tumor.
Case Report
A 65-year-old man presented himself to the Emergency Department of the Constanta County Emergency Clinical Hospital for upper digestive hemorrhage expressed in the form of hematemesis, with approximately 300 mL of fresh to altered blood, and with a history of melena. He was admitted to the Gastroenterology Department for additional investigations.
From the pathological antecedents, multiple cardiovascular diseases were noted, such as essential arterial hypertension, mitral insufficiency, ectasia of the ascending aorta, and New York Heart Association III heart failure stage C. To these were added chronic obstructive bronchopathy and dyspepsia.
The clinical examination revealed a temporospatially oriented patient with orthopnea, absence of pathological rales, systolic murmur in the mitral focus, body mass index of 32.4, and a painful upper abdomen on deep palpation.
Paraclinical examinations identified erythropenia (red blood cells, 2.38×106/μL), low hematocrit level (29.9%), low hemoglobin level (6.6 g/dL), normal mean corpuscular volume (82.4 fl), normal mean corpuscular hemoglobin (27.6 pg), normal mean corpuscular hemoglobin concentration (32.8 g/dL), leukocytosis with neutrophilia (leukocyte count, 19.63×103/μL and neutrophil count, 13.96×103/μL), hyperglycemia (glucose, 358.50 mg/dL), hyper-creatinemia (creatinine, 2.43 mg/dL), increased serum urea (219 mg/dL), low international normalized ratio (1.12), and hyperkalemia (potassium, 6.4 mmol/L).
Upper digestive endoscopy revealed a subepithelial lesion at the antral level, with an ulcerated center and rare adherent blood clots. Biopsy fragments could not be taken from this level.
Computed tomography (CT) with contrast enhancement highlighted a thickening of the gastric mucosa at the level of the submucosa of the antral region, an oval area with approximate dimensions of 28×16 mm. The contrast uptake was natively dense and heterogeneous (Figure 1). These were associated with a small hiatal hernia through sliding of the cardia with intrathoracic ascension. Tumor adenopathies and intraperitoneal fluid were not evident.
Surgery was performed through gastrotomy, excision of the antral formation, and gastrorrhaphy. The presumptive diagnosis was gastrointestinal stromal tumor. The postoperative evolution was favorable, with present intestinal transit and good digestive tolerance.
The macroscopic examination revealed a tissue fragment measuring 2.5×2×2 cm with a smooth external surface, partially covered by the gastric mucosa with discrete superficial ulcerations covered by blood clots. On section, the lesion was solid, greyish-brown in color, and inhomogeneous in appearance (Figure 2).
Microscopically, in the thickness of the gastric wall, a non-encapsulated lesion with a maximum diameter of 1.7 cm was seen. It was located at the level of the muscularis propria, whose smooth muscle fibers it dissected, and it elevated the mucosa without invading it. However, it produced its ulceration, and at this level, fibrino-hematic deposits and cellular debris were identified. The lesion was composed of a proliferation of medium-sized, round-oval cells, with reduced cytoplasm; it was weakly eosinophilic, with round nuclei with finely granular chromatin (Figure 3). The architecture was solid and focally arranged in a nest, and the stroma was fibro-hyalinized. Within the proliferation, vascular spaces with variable sizes and shapes were identified, in some places with blood clots.
Immunohistochemical tests excluded the presumptive diagnosis. The final diagnosis was a benign mesenchymal tumor originating in the perivascular glomus body, a glomus tumor (Table 1, Figures 4, 5).
At discharge, the erythrocyte count reached 2.38×106/μL, and the hemoglobin level reached 10.3 g/dL. The other biochemical values of leukocytes, blood glucose, serum creatinine, urea, and serum potassium normalized.
Discussion
The challenging task faced by pathologists lies in deciphering the deceptive nature of this rare tumor, as glomus tumor poses difficulties both in terms of imaging and its macroscopic appearance. Although a benign tumor can be seemingly harmless, its clinical manifestations can still have potentially life-threatening consequences for the patient. Therefore, a good knowledge of all the entities that make the differential diagnosis, combined with the histopathological and immunohistochemical examinations, must be the tools of any doctor.
Glomus tumors were first described in 1924 by Masson et al. Four years later, Tliejeva et al performed the description of a glomus tumor at the gastric level [7]. The prevalence of these subepithelial lesions is rarely reported, with studies to date reporting rates between 0.8% and 1.7% [6]. Localization in the gastrointestinal tract is rare. Most of the time, in the gastric location, glomus tumors are identified in the submucosa or muscularis of the antrum, and at the intestinal level, they are found in the wall of the cecum [8,9].
There is no specific decade of life in which they appear, with cases being reported in 19-year-old and 90-year-old patients; however, the average patient age is 55 years. Also, a strong female predominance has been observed [10]. In the presented case, the patient was male and had an older age.
In most cases of glomus tumor, patients are asymptomatic. In symptomatic cases, patients report epigastric pain, nausea, vomiting, ulcer syndrome, and, rarely, upper digestive hemorrhage [11,12]. The manifestation of upper digestive hemorrhage was also the reason why our patient presented to the doctor. Unlike our case and most of the data in the literature, in the report by Papaelis A et al, the patient presented an atypical symptomology represented by a persistent cough and shortness of breath [13].
Asymptomatic cases can be detected accidentally during a gastrointestinal endoscopy [14]. The most common diagnostic methods consist of endoscopic ultrasonography and CT. Endoscopic ultrasonography reveals well-defined masses with frequent localization in the third or fourth gastric layers (submucosa and muscularis propria, respectively), with predominantly hypoechoic appearance with hyperechoic areas, due to its vascular nature [15–17]. Aspects that can suggest malignancy are represented by irregular edges, cystic or necrotic areas, and echogenic areas [15].
CT provides valuable information regarding size, morphology, internal structure, type of growth, and blood supply [18]. CT without contrast usually reveals a single lesion located in the gastric wall with endophytic and/or exophytic growth. In contrast-enhanced CT, an improvement is observed in the arterial phase, which also persists in the venous phase. Also, the lesion is well-defined with early and heterogeneous contrast uptake [4,18]. However, sometimes the CT can be exceeded, as happened in the case of Mehmood et al. The failure of the imaging diagnosis was attributed to insufficient gastric distension, which did not allow visualization of the lesion [19]. Rarely, a magnetic resonance imaging examination can be performed, identifying a hypointense mass in T1-weighted and hyperintense mass in T2-weighted sequences [4].
Endoscopic ultrasonography and CT cannot establish clear diagnostic criteria. Thus, techniques such as fine needle aspiration or endoscopically guided fine needle biopsy help in a correct, minimally invasive diagnosis [15]. On the aspirated smears, clusters or nests of low-moderate, uniform, round to polygonal cells with pale, reduced cytoplasm and central, round-oval nuclei with fine chromatin with small, occasional nucleoli are observed [20]. Fine needle biopsy is an important method of diagnosis, but its accuracy decreases the deeper the lesion is located [14].
Both for treatment and for correct diagnosis, resection with free margins is preferred. A minimally invasive intervention is desired. The most used procedures are wedge resection, subtotal gastrectomy, or tumor resection [21,22]. In small lesions, it is possible to opt for an excision through endoscopy, but the risks of hemorrhage and perforation must be weighed. Besides these, the endoscopic treatment becomes unnecessary if the margins are positive [23]. In the case of lymphadenectomy, there is no consensus, as some authors do not consider it routine [21].
Macroscopically, the lesions have an average size between 2 and 3 cm and are solitary with well-defined edges. On the section, they have a grayish-reddish color, are of slightly increased consistency, and can be associated with hemorrhage or calcification [7].
Three variants can be observed histopathologically. The solid form (75%) is composed of nests and islands of glomerular cells surrounded by capillaries and hyalinized stroma or with myxoid changes. Glomangioma (20%) is a form in which a venous proliferation (cavernous hemangioma appearance) is observed surrounded by nests of glomus cells. Glomangiomyoma (5%) is characterized by the presence of elongated glomus cells, with an appearance similar to smooth muscle cells [2,24].
There are cases in which these tumors can become malignant. Due to the rarity, the criteria are not stated and are based on the research of Folpe et al, which consists of deep localization and dimensions over 20 mm, the presence of atypical mitotic figures, and the association between a moderate to high nuclear grade, with a mitotic activity of more than 5 mitoses/50 HPF high-power fields [25]. In malignant cases, the most frequent metastases are at the pulmonary, mediastinal, cerebral, or hepatic levels. As a rule, in metastatic cases, the condition is one with an increased lethality in a very short time [26].
From a cytogenetic point of view, the CARMN: : NOTCH2 gene fusion is frequently noted. biallelic neurofibromatosis 1 (NF1) inactivation or glomulin (GLMN) gene inactivation can be identified in familiar cases. Occasionally, Kirsten rat sarcoma virus (KRAS) and V-Raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations can be seen, the latter being associated with malignancy [4].
The most important aspect of this pathology, both at the time of curative interventions and during the histopathological examination, is the differential diagnosis. Thus, the pathologist has two challenges in front of him, to differentiate proliferation from other mesenchymal lesions and to establish its biological potential [27].
The main entities with which differential diagnosis is made are summarized in Table 2 [27–31].
Conclusions
The deceptive appearance that such a rare tumor can create from an imaging point of view (endoscopy and CT), but also macroscopically, represents a challenge for the pathologist. Histopathological and especially immunohistochemical examinations are essential for the correct diagnosis of glomus tumor, as well as for establishing the biological potential. Even if it is a benign tumor, the effects it produces through the clinical picture can put the patient’s life in danger. For this reason, adequate management of the case, which requires a good knowledge of all the entities that enter into the differential diagnosis, is an essential goal to be achieved for saving lives.
Figures
References:
1.. Ezeh KJ, Boateng W, Paudel B, A case of gastric glomus tumor misdiag-nosed as carcinoid tumor: Cureus, 2023; 15(1); e34316
2.. Güzel FA, Göksu M, Örmeci A, Stomach glomus tumor: New Trends Med Sci, 2020; 1(1); 46-50
3.. Vyawahare MA, Musthyala BN, Tayade RT, Gastric glomus tumor: A rare etiology of upper gastrointestinal bleed: Indian J Pathol Microbiol, 2021; 64(4); 795-98
4.. Zironda A, Grotz TE, Folpe AL, Thiels CA, Gastrointestinal glomus tumors: A single institution, 20-year retrospective study: J Surg Res, 2023; 283; 982-91
5.. Tantia M, Suryawanshi PR, Gupta A, Rachakatla P, Gastric glomus tumour: A case report: J Minim Access Surg, 2021; 17(4); 551-53
6.. Choe Y, Cho YK, Kim GH, Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: A multicenter study: Clin Endosc, 2023; 56(6); 744-53
7.. Deng M, Luo R, Huang J, Clinicopathologic features of gastric glomus tumor: A report of 15 cases and literature review: Pathol Oncol Res, 2023; 28; 1610824
8.. Miettinen M, Paal E, Lasota J, Sobin LH, Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases: Am J Surg Pathol, 2002; 26(3); 301-11
9.. Tsagkataki ES, Flamourakis ME, Gkionis IG, Gastric glomus tumor: A case report and review of the literature: J Med Case Rep, 2021; 15(1); 415
10.. , 2019, Lyon, International Agency for Research on Cancer
11.. Singh S, Kumar A, Singh V, Gastric glomus tumor.: Niger J Surg, 2020; 26(2); 162-65
12.. Toti L, Manzia TM, Roma S, Rare malignant glomus tumor of the stomach with liver metastases: Radiol Case Rep, 2019; 14(4); 463-67
13.. Papadelis A, Brooks CJ, Albaran RG, Gastric glomus tumor.: J Surg Case Rep., 2016; 2016(11) rjw183
14.. Alsahwan AG, Alfaraj ZM, AlSafwani J, Rare gastric neoplasm: Malignant glomus tumor of the stomach. A case report.: Int J Surg Case Rep, 2021; 81; 105802
15.. Kang G, Park HJ, Kim JY, Glomus tumor of the stomach: A clinicopathologic analysis of 10 cases and review of the literature: Gut Liver, 2012; 6(1); 52-57
16.. Hu J, Ge N, Wang S, The role of endoscopic ultrasound and endoscopic resection for gastric glomus: A case series and literature review: J Transl Int Med, 2019; 7(4); 149-54
17.. Bai B, Mao CS, Li Z, Kuang SL, Endoscopic ultrasonography diagnosis of gastric glomus tumors: World J Clin Cases, 2021; 9(33); 10126-33
18.. Xing JJ, Huang WP, Wang F, Computed tomography features and clinicopathological characteristics of gastric glomus tumor: BMC Gastroenterol, 2022; 22(1); 174
19.. Mehmood F, Jamil H, Khalid A, Gastric glomus tumor: A rare cause of acute blood loss anemia: Cureus, 2022; 14(4); e24511
20.. Farooq A, Goyal A, Giorgadze T, Cytomorphological features of glomus tumors arising in the stomach: A series of two cases diagnosed on FNA: Ann Diagn Pathol, 2019; 42; 42-47
21.. Antony P, Renzulli MM, Vrugt B, Glomus tumor of the stomach. Diagnostic approach and surgical management.: Clin Onco, 2022; 6(16); 1-4
22.. Ayash A, Elkomy N, Al-Mohannadi MJ, Gastric glomus tumor presenting with massive upper GI bleeding: A challenging to diagnose and treat tumor: Clin Case Rep, 2022; 10(8); e6172
23.. Wang WH, Shen TT, Gao ZX, Combined laparoscopic-endoscopic approach for gastric glomus tumor: A case report: World J Clin Cases, 2021; 9(24); 7181-88
24.. Mohamed WT, Jahagirdar V, Jaber F, Glomus tumor of the stomach presenting with upper gastrointestinal bleeding: A case report: J Investig Med High Impact Case Rep, 2023; 11 23247096231192891
25.. Hasuda H, Hu Q, Miyashita Y, Gastric glomus tumor with a preoperative diagnosis by endoscopic ultrasonography-guided fine needle aspiration: A case report: Int Cancer Conf J, 2020; 10(1); 35-40
26.. Wang S, Ding C, Tu J, Malignant glomus tumor of the lung with multiple metastasis: A rare case report: World J Surg Oncol, 2015; 13; 22
27.. Mago S, Pasumarthi A, Miller DR, The two challenges in management of gastric glomus tumors: Cureus, 2020; 12(7); e9251
28.. Sbaraglia M, Businello G, Bellan E, Mesenchymal tumours of the gastrointestinal tract: Pathologica, 2021; 113(3); 230-51
29.. Wang X, Hanif S, Wang B, Chai C, Management of gastric glomus tumor: A case report: Medicine (Baltimore), 2019; 98(38); e16980
30.. Xu J, Yan Y, Xiang X, Gastric perivascular epithelioid cell tumor (PEComa): Am J Clin Pathol, 2019; 152(2); 221-29
31.. Bi YZ, Yan SJ, Zhou LM, A rare case of gastrointestinal bleeding caused by gastric capillary hemangioma.: Gastroenterol Rep (Oxf)., 2023; 11 goad026
Figures
Tables
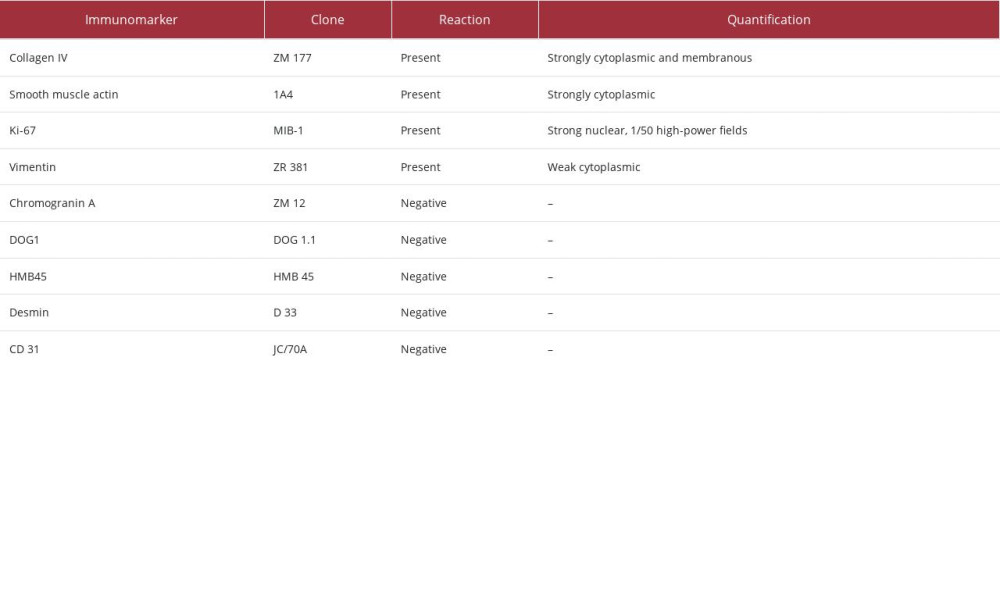 Table 1.. Interpretation of immunohistochemistry performed for the diagnosis of glomus tumor.
Table 1.. Interpretation of immunohistochemistry performed for the diagnosis of glomus tumor.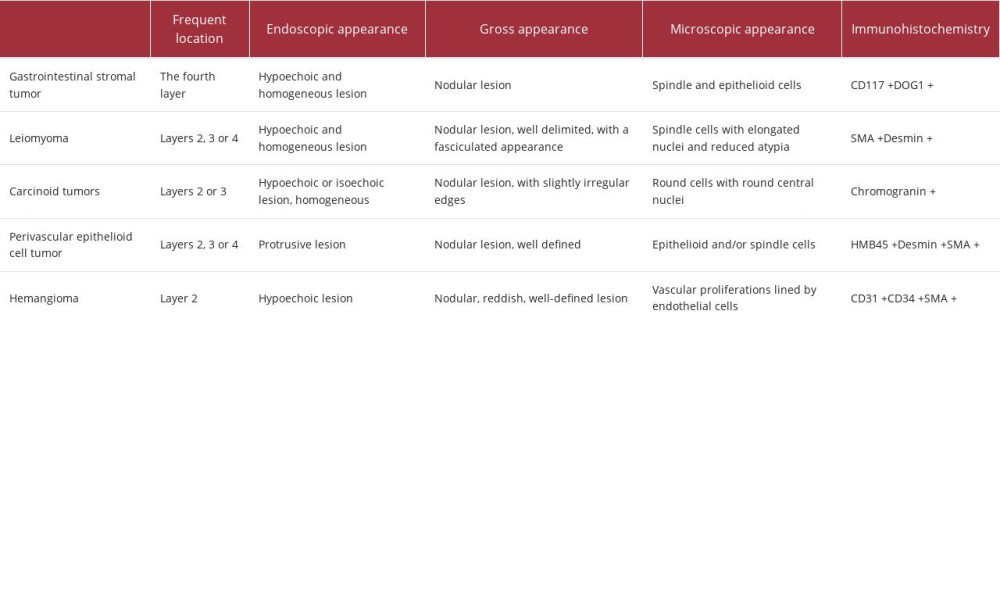 Table 2.. The main entities from which a gastric glomus tumor must be differentiated [27–31].
Table 2.. The main entities from which a gastric glomus tumor must be differentiated [27–31]. Table 1.. Interpretation of immunohistochemistry performed for the diagnosis of glomus tumor.
Table 1.. Interpretation of immunohistochemistry performed for the diagnosis of glomus tumor. Table 2.. The main entities from which a gastric glomus tumor must be differentiated [27–31].
Table 2.. The main entities from which a gastric glomus tumor must be differentiated [27–31]. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








