30 January 2024: Articles 
Uncovering Primary Extranodal Diffuse Large B Cell Lymphoma in the Adrenal and Thyroid Glands
Unusual clinical course, Challenging differential diagnosis, Rare disease
Judy LiDOI: 10.12659/AJCR.942659
Am J Case Rep 2024; 25:e942659
Abstract
BACKGROUND: Primary extranodal diffuse large B-cell lymphoma (DLBCL) is a rare, yet highly aggressive and invasive malignancy that can masquerade as a solid organ tumor. Timely diagnosis is critical for improving prognosis; however, it is challenging to achieve.
CASE REPORT: We report 2 cases treated at Yale New Haven Hospital (New Haven, CT, USA) and the West Haven Veteran’s Affairs Medical Center (West Haven, CT, USA) in 2023. Case 1 describes a 69-year-old woman who presented with a large left adrenal mass that was suspicious for adrenocortical carcinoma and was found to have primary adrenal DLBCL following surgical resection. Case 2 describes a 59-year-old woman with Hashimoto’s thyroiditis and goiter who was found to have primary thyroid DLBCL following partial thyroidectomy.
CONCLUSIONS: Primary extranodal DLBCL should be included in the differential diagnosis of solid adrenal and thyroid tumors. The risks of biopsy, given currently available techniques, should be weighed against the benefits of achieving a definite diagnosis, allowing for timely initiation of systemic immunochemotherapy. When biopsy can be safely performed, techniques designed to evaluate for DLBCL should be incorporated.
Keywords: Adrenal Gland Neoplasms, delayed diagnosis, Lymphoma, Non-Hodgkin, surgical oncology, Thyroid Neoplasms
Background
Diffuse large B-cell lymphoma (DLBCL) is an aggressive hematopoietic malignancy that is diagnosed by its morphologic, immunophenotypic, and molecular profile. Patients most commonly present with a symptomatic lymphadenopathy, in which core or excisional lymph node biopsy is subsequently performed [1]. The average age of diagnosis is 64 years, and approximately 30% of patients experience B symptoms, which include fever, night sweats, and weight loss [2]. Roughly one-third of cases arise from extranodal sites (most commonly the gastrointestinal tract or skin [3]); although, in cases where extranodal and nodal disease coexist, it is challenging to differentiate between primary extranodal and nodal disease. The definition of primary extranodal DLBCL has remained controversial, but it is currently accepted to classify DLBCL as primary extranodal when the extranodal component is clinically dominant and there is no to minimal nodal involvement or history of lymphoma [4]. However, in rare cases, DLBCL can present as a solid organ tumor and masquerade as a carcinoma [5,6] or sarcoma [7] on imaging, especially when symptoms are nonspecific. Biopsies may thus not be routinely performed out of concern for malignancies in which tissue sampling prior to surgical resection is not indicated, such as in the first case described here of an adrenal mass suspicious for adrenocortical carcinoma. If biopsies are performed, such as in our second case, of an enlarging thyroid, standard techniques may not gather significant enough tissue or incorporate cytology techniques optimized for lymphoma detection, decreasing the likelihood of an accurate and timely diagnosis. The aim of this report is to share 2 cases of primary extranodal DLBCL presenting as solid organ tumors: the first being a case of primary adrenal DLBCL and the second being a case of primary thyroid DLBCL. Both cases presented with minimal lymphadenopathy in the absence of B symptoms and were not known to be DLBCL until after surgical resection.
Case Reports
CASE 1:
A 69-year-old woman presented to the Emergency Department with severe, worsening left upper quadrant and epigastric abdominal pain upon waking up that morning. The pain radiated to the left flank and pelvis. Her symptoms included intermittent hot flashes and nausea; she denied gastrointestinal symptoms (vomiting, diarrhea, changes in appetite), B symptoms (fever, chills, night sweats), weight loss, chest pain, shortness of pain, or any genitourinary symptoms. She had been experiencing burning back pain for 8 months, which she controlled with over-the-counter pain medications, as well as nail ridging and hair loss. She did not initially seek care, owing to lack of insurance. However, she presented for care due to worsening abdominal and flank pain that was no longer responsive to pain medications.
Computed tomography (CT) scan with contrast of the abdomen and pelvis revealed a large (7.1×6.6×8.3 cm) left heterogeneous, centrally hypodense and possibly necrotic mass in the left retroperitoneal space, with associated lymphadenopathy (eg, 2.2-cm left para-aortic lymph node). The mass appeared to be inseparable from the left kidney and adrenal gland and appeared to invade the left diaphragm (Figure 1). Follow-up CT scan of the chest and head did not reveal evidence of metastatic disease or additional lymphadenopathy.
Magnetic resonance imaging (MRI) of the abdomen was additionally performed and identified an enhancing mass favored to be of left adrenal origin. A normal left adrenal gland was not identified, and the mass and its associated retroperitoneal lymphadenopathy appeared to encase the left renal artery and vein and abut the aorta and the origin of the celiac and superior mesenteric arteries (Figure 2).
Surgical oncology, endocrine surgery, and urology services were consulted for initial workup. As reported in Table 1, blood testing revealed mild transaminitis and slightly low dehydroepiandrosterone sulfate. A negative dexamethasone suppression test and normal levels of thyroid stimulating hormone, adrenocorticotropic hormone, plasma and 24-h urine epinephrine and metanephrines, aldosterone, and plasma renin activity suggested that the mass was likely non-functional.
Her past medical history was notable for hypertension and hypothyroidism, managed with losartan and levothyroxine, respectively. Her past surgical history included a hysterectomy and salpingo-oophorectomy, due to concern for possible cancer several years ago, but the final pathology report did not demonstrate any malignancy. She denied any family history of cancer and prior exposure to radiation; however, she was exposed to toxic chemicals while working as a housekeeper prior to retiring.
Given concern for locally advanced adrenocortical carcinoma, resection was planned and performed by a multidisciplinary team including endocrine, oncologic, thoracic, urological, and vascular surgeons. Out of concern for tumor seeding, a preoperative diagnostic biopsy was not undertaken. The patient underwent an open left adrenalectomy with en-bloc nephrectomy, para-aortic lymph node dissection, and partial diaphragm resection and repair. A large, firm multinodular mass involving the left adrenal gland, left upper pole of the kidney, left renal hilum, and diaphragm with para-aortic adenopathy was confirmed intraoperatively. The mass encased the 2 left renal arteries and adhered to, but did not invade, the superior mesenteric artery, celiac artery, and abdominal aorta.
The en-bloc resection specimen (weighing 803 g and measuring 18×10×9 cm), including the kidney, adrenal gland, and a portion of diaphragm. A diagnosis of DLBCL, non-germinal center sub-type, was confirmed by morphologic evaluation and immunophenotypic analysis. The lesion, measuring 8.7×8.5×8.2 cm in size, consisted of a tan, bulky mass with a “fish-flesh” appearance (Figure 3). Microscopy and histopathological examination of the mass revealed effacement of normal renal parenchyma by a diffuse proliferation of large lymphocytes with vesicular chromatin and variably prominent nucleoli. Increased mitotic activity with numerous apoptotic bodies and frank necrosis were also identified (Figure 4A). The tumor was cell CD20(+), CD10(−), CD30(−), CD138(−), MUM-1(+), BCL-2(+), BCL-6(+), and Ki-67(50%) (Figure 4B–4D). Central Nervous System International Prognostic Index score was 4. The fluorescence in situ hybridization (FISH) panel was negative for c-MYC gene rearrangements.
As reported in Table 2, HIV and hepatitis panels were negative. Postoperative serum beta-2 microglobulin was elevated at 3.69 mg/L, and lactate dehydrogenase (LDH) was elevated at 309 in a slightly hemolyzed sample. The 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan demonstrated residual retroperitoneal metabolically active disease without splenomegaly. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) therapy with intrathecal methotrexate and growth factor support were initiated.
CASE 2:
A 59-year-old woman with hypothyroidism secondary to Hashimoto thyroiditis presented with right neck swelling that seemed to subside following resolution of a viral infection, only to return and lead to compressive symptoms of voice hoarseness, morning cough, orthopnea, difficulty swallowing, and a positive Pemberton sign. She also developed predominantly left-sided neck tenderness. Other past medical history included hypertension, hyperlipidemia, gastroesophageal reflux disease, nephrolithiasis, and vitamin D deficiency. She had a right thyroid cyst drained 10 years ago but otherwise had no prior surgeries. She had no personal or family history of cancer. A CT scan of the head and neck demonstrated a diffusely enlarged thyroid gland. A subsequent thyroid ultrasound revealed an enlarging left thyroid gland with left cervical lymph-adenopathy. The right thyroid lobe was 3.2×6.2×2.6 cm (volume: 26.8 mL, increased from 20 mL 8 years ago), and the left thyroid lobe was 5.2×7.2×4.3 cm (volume: 83 mL, increased from 14 mL) with an enlarged cervical lymph node measuring 1.8×1.8×1.7 cm. Both lobes were heterogeneous, diffusely hypoechoic without nodules and with hyperemic changes (mild on right; moderate on left).
Because of the patient’s extremely tender left neck and severe anxiety regarding biopsy of this area, as well as the lack of any discrete nodules within the gland, biopsy of the right thyroid lobe with fine needle aspiration (FNA) was performed. Cytologic evaluation revealed clusters of follicular cells with mixed lymphocytes, consistent with thyroiditis. As reported in Table 3, thyroid function test results were within normal limits, consistent with the known history of Hashimoto thyroiditis and levothyroxine supplementation.
A total thyroidectomy was planned for compressive symptoms. During the operation, a large necrotic left thyroid lobe with extensive encasement of the trachea and carotid sheath structures was noted. Thus, a left partial thyroidectomy was completed for diagnostic purposes. Microscopy and histopathological examination of the resected specimen revealed a diffuse infiltrate of atypical cells. The cells were intermediate to large in size, with irregular nuclear contours and a scant to moderate amount of cytoplasm (Figure 5A). One section revealed intact foci of thyroid follicles without lymphoplasmacytic infiltrates or lymphoepithelial lesions. The malignant cells were CD20(+), PAX-5(+), CD10(−), CD3(−), CD5(−), CD138(−), CYCLIN-D1(−), MUM-1(−), BCL-2(+), BCL-6(+), and Ki-67(80%) (Figure 5B–5D). Additionally, there was no light-chain restriction by immunohistochemistry. The morphologic and immunohistochemistry features failed to rule in an extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type or definite MALT transformation. The FISH panel was negative for BCL2 and BCL6 gene rearrangements but equivocal for c-MYC. This constellation of findings confirmed a diagnosis of DLBCL, germinal cell subtype.
Postoperative laboratory test results revealed elevated LDH but were otherwise noncontributory (Table 4). An 18F-FDG PET/CT scan demonstrated residual hypermetabolic activity in the remaining thyroid gland and bed. Pola-R-CHP (polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, and prednisone) therapy was initiated with prophylactic allopurinol, trimethoprim-sulfamethoxazole, and valacyclovir.
Discussion
Primary adrenal DLBCL and primary thyroid DLBCL are extremely rare malignancies, each accounting for less than 2% to 3% of all primary extranodal lymphomas [8,9]. Both DLBCL cases described in this report presented as symptomatic, enlarging non-functional solid organ tumors with minimal associated lymphadenopathy and without B symptoms. Diagnosis of DLBCL was not reached until after surgical resection was performed.
In case 1, preoperative biopsy was not conducted due to concern for adrenocortical carcinoma. Current guidelines recommend against biopsy of large adrenal masses suspicious for malignancy, such as adrenocortical carcinoma, given inevitable resection in most cases, limited diagnostic utility, and risks for complications and needle-track seeding [10,11]. The exception to this rule is when an adrenal mass requires a treatment other than surgical resection, such as in the case of lymphoma requiring immunochemotherapy. In practice, this distinction between adrenocortical carcinoma and DLBCL can be extremely challenging to discern with confidence, given the heterogeneity of presentation. Preoperative biopsy of adrenal masses has been generally avoided because of the aforementioned risks; however, recent advances in image-guided techniques have improved biopsy safety without compromising tissue quality [12,13] and may have the potential to change guidelines in the future.
In contrast, preoperative FNA remains the universal criterion standard first-line diagnostic tool for thyroid malignancies and thus was performed in case 2. However, FNA failed to reveal DLBCL and only demonstrated Hashimoto disease. Resection was ultimately performed for compressive symptoms, and only then was DLBCL revealed on tissue pathology. Thyroid FNA appears to have decent diagnostic accuracy in detecting malignancy, with sensitivities ranging from 65% to 98% and specificities from 72% to 100% [14]. Unfortunately, diagnostic performance of FNA is markedly poorer in cases of lymphoma due to limited tissue sampling [15,16], and thus, more generous core, if not excisional, biopsies are recommended over FNA when lymphoma of the thyroid is suspected [16]. Different cytology tests also have varying degrees of sensitivity for detecting atypical cells. In practice, cell block preparations are selected based on clinical impression, which in this case was goiter without nodules and Hashimoto thyroiditis, and thus a cell block preparation not designed for the evaluation of lymphoma (ie, one with significant artifact and low cellularity) was used. Finally, the FNA was performed on the less tender and less diseased side of the thyroid, which may have further reduced the likelihood of lymphoma detection.
Consideration of DLBCL is important in the evaluation of all solid organ tumors because DLBCL must be treated with systemic immunochemotherapy as opposed to solely surgical resection. Assessment of DLBCL likelihood in such tumors can help weigh the risks and benefits of preoperative biopsy, which is required for definitive diagnosis, but is challenging. In the absence of systemic B symptoms, which is the case among the vast majority of patients presenting with DLBCL, the clinical presentation of DLBCL can be especially difficult to differentiate from those of other malignancies. Common local symptoms of primary adrenal or primary thyroid lymphoma (eg, abdominal pain and symptomatic goiter, respectively) are highly nonspecific. Elevated LDH could reflect DLBCL but is also nonspecific and does not rule out other disease processes [17]. Elevated serum beta-2 microglobulin and atypical cells on peripheral smears could similarly increase suspicion for a lymphoproliferative disorder such as DLBCL; however, they are currently not routinely ordered in the workup of solid organ tumors (and were not ordered in either of these cases prior to diagnosis).
However, as more cases of primary adrenal and primary thyroid DLBCL are reported and studied, patterns may emerge and help guide diagnostic workup. The largest systematic review of primary adrenal lymphoma to date, consisting of 187 cases, found that most cases were among elderly men (with a 1.8: 1 male-to-female ratio), involved bilateral adrenal glands (75%), and presented with adrenal insufficiency (61%) and elevated LDH (88%) [18]. More recent studies of case series have yielded similar findings [19–21], although the largest series of 97 patients found the distribution between bilateral and unilateral adrenal involvement to be more evenly split [21]. Despite these emerging associations, the clinical spectrum of primary adrenal lymphoma appears vast, and current retrospective studies have been limited by heterogeneity in the data collected and reported. On the other hand, primary thyroid lymphomas appear to have a greater predominance in elderly women (with a 3: 1 female-to-male ratio [22]). In addition, Hashimoto thyroiditis has been associated with an estimated 60-fold increase in risk for thyroid lymphoma [16,23], although it is more commonly associated with papillary thyroid carcinoma [24]. Interestingly, calcifications have been a common finding on ultrasound and CT imaging in thyroid carcinoma [25] but uncommon in thyroid lymphoma [26]. Overall, these associations alone remain inadequate in discriminating lymphoma from other pathologies without biopsy. Further work can be directed toward identifying profiles of risk factors and clinical attributes that may increase suspicion for lymphoma and therefore inform decision-making, namely, whether or not to perform a biopsy in light of risks associated with comorbidi-ties and other diseases on the differential, amount of tissue to biopsy, cell block preparation methods.
The cases described in this report were consistent with the proposed classic presentation of primary thyroid lymphoma but not that of primary adrenal lymphoma, which is unsurprising given the greater clinical heterogeneity of the latter disease. Surgical resection was most likely inevitable due to mass effect symptoms. Given that the resected tissue pathology would yield a definitive diagnosis, efforts could be directed toward expediting an excisional tumor biopsy when lymphoma is suspected and if surgery is to be inevitably performed for symptom management. However, if not expedited, postponement of surgery can delay diagnosis and initiation of necessary chemotherapy. Prior studies have also failed to identify any survival benefit from surgical resection in primary adrenal [19] and primary thyroid DLBCL [9], unless supplemented with systemic chemoimmunotherapy (eg, R-CHOP or pola-R-CHP) at early stages [27]. Thus, the impact of a potential delay in diagnosis and treatment in the absence of a preoperative biopsy should be carefully considered if surgical resection is pursued. In the case of smaller or asymptomatic masses not requiring immediate resection for symptomatic relief, a preoperative biopsy can be beneficial if it can be performed safely. Current guidelines for DLBCL diagnosis recommend an excisional lymph node biopsy over core biopsy or FNA, which are more susceptible to false negatives or inconclusive results due to limited sampling [1,28]. However, when primary extranodal disease is being considered, lymph nodes may or may not be involved, and thus, lymph node biopsy can be required. Benefits and risks of the procedure differs based on disease burden, safety of biopsy given the target tissue and available techniques, tissue sample quantity and quality, timing of potential resection, and the types of malignancies being considered.
Conclusions
As discussed in this brief 2-case series, primary adrenal DLBCL and primary thyroid DLBCL are rare yet aggressive hematopoietic malignancies that require histopathology review for diagnosis. Given clinical presentations that overlap with solid organ tumors suggestive of other malignancies, such as thyroid carcinoma and adrenocortical carcinoma, biopsies with a large enough sample and sensitive cytology techniques may not be performed prior to surgical resection, leading to delays in diagnosis and initiation of necessary systemic immunochemotherapy.
Figures
References:
1.. Liu Y, Barta SK, Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment: Am J Hematol, 2019; 94(5); 604-16
2.. Matasar MJ, Zelenetz AD, Overview of lymphoma diagnosis and management: Radiol Clin North Am, 2008; 46(2); 175-98
3.. Ollila TA, Olszewski AJ, Extranodal diffuse large B cell lymphoma: Molecular features, prognosis, and risk of central nervous system recurrence: Curr Treat Options Oncol, 2018; 19(8); 38
4.. Zucca E, Extranodal lymphoma: A reappraisal: Ann Oncol, 2008; 19(Suppl. 4); iv77-80
5.. Parakh R, Cheng L, Tretiakova M, PAX8-positive B-cell lymphoma in adrenal gland masquerading as metastatic renal cell carcinoma: Int J Surg Pathol, 2018; 26(8); 721-24
6.. Alzerwi NAN, Primary pancreatic lymphoma masquerading as carcinoma: Case Rep Oncol Med, 2020; 2020; 5160545
7.. Elkourashy SA, Nashwan AJ, Alam SI, Aggressive lymphoma “sarcoma mimicker” originating in the gluteus and adductor muscles: A case report and literature review: Clin Med Insights Case Rep, 2016; 9; 47-53
8.. Kim YR, Kim JS, Min YH, Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemo-therapy from the Consortium for Improving Survival of Lymphoma (CISL): J Hematol Oncol, 2012; 5; 49
9.. Yi J, Yi P, Wang W, A multicenter retrospective study of 58 patients with primary thyroid diffuse large B cell lymphoma: Front Endocrinol (Lausanne), 2020; 11; 542
10.. Williams AR, Hammer GD, Else T, Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome: Eur J Endocrinol, 2014; 170(6); 829-35
11.. Shariq OA, McKenzie TJ, Adrenocortical carcinoma: Current state of the art, ongoing controversies, and future directions in diagnosis and treatment: Ther Adv Chronic Dis, 2021; 12; 20406223211033103
12.. Kandel P, Tranesh G, Nassar A, EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study: Gastrointest Endosc, 2016; 84(6); 1034-39
13.. Tyng CJ, Bitencourt AG, Martins EB, Technical note: CT-guided paravertebral adrenal biopsy using hydrodissection – a safe and technically easy approach: Br J Radiol, 2012; 85(1015); e339-42
14.. Hambleton C, Kandil E, Appropriate and accurate diagnosis of thyroid nodules: A review of thyroid fine-needle aspiration: Int J Clin Exp Med, 2013; 6(6); 413-22
15.. Karimi-Yazdi A, Motiee-Langroudi M, Saedi B, Diagnostic value of fine-needle aspiration in head and neck lymphoma: A cross-sectional study: Indian J Otolaryngol Head Neck Surg, 2014; 66(Suppl. 1); 277-80
16.. Lee JS, Shin SJ, Yun HJ, Primary thyroid lymphoma: A single-center experience: Front Endocrinol (Lausanne), 2023; 14; 1064050
17.. Forkasiewicz A, Dorociak M, Stach K, The usefulness of lactate dehydrogenase measurements in current oncological practice: Cell Mol Biol Lett, 2020; 25; 35
18.. Rashidi A, Fisher SI, Primary adrenal lymphoma: A systematic review: Ann Hematol, 2013; 92(12); 1583-93
19.. Laurent C, Casasnovas O, Martin L, Adrenal lymphoma: Presentation, management and prognosis: QJM, 2017; 110(2); 103-9
20.. Wang Y, Ren Y, Ma L, Clinical features of 50 patients with primary adrenal lymphoma: Front Endocrinol (Lausanne), 2020; 11; 595
21.. Majidi F, Martino S, Kondakci M, Clinical spectrum of primary adrenal lymphoma: Results of a multicenter cohort study: Eur J Endocrinol, 2020; 183(4); 453-62
22.. Graff-Baker A, Roman SA, Thomas DC, Prognosis of primary thyroid lymphoma: Demographic, clinical, and pathologic predictors of survival in 1,408 cases: Surgery, 2009; 146(6); 1105-15
23.. Holm LE, Blomgren H, Lowhagen T, Cancer risks in patients with chronic lymphocytic thyroiditis: N Engl J Med, 1985; 312(10); 601-4
24.. Hussein O, Abdelwahab K, Hamdy O, Thyroid cancer associated with Hashimoto thyroiditis: Similarities and differences in an endemic area: J Egypt Natl Canc Inst, 2020; 32(1); 7
25.. Yang TT, Huang Y, Jing XQ, CT-detected solitary thyroid calcification: An important imaging feature for papillary carcinoma: Onco Targets Ther, 2016; 9; 6273-79
26.. Peng C, Yi D, Zhou Y, Differential diagnosis of non-diffuse primary thyroid lymphoma and papillary thyroid carcinoma by ultrasound combined with computed tomography: BMC Cancer, 2022; 22(1); 938
27.. Schmitz C, Rekowski J, Muller SP, Impact of complete surgical resection on outcome in aggressive non-Hodgkin lymphoma treated with immunochemotherapy: Cancer Med, 2020; 9(22); 8386-96
28.. Syrykh C, Chaouat C, Poullot E, Lymph node excisions provide more precise lymphoma diagnoses than core biopsies: A French Lymphopath network survey: Blood, 2022; 140(24); 2573-83
Figures
Tables
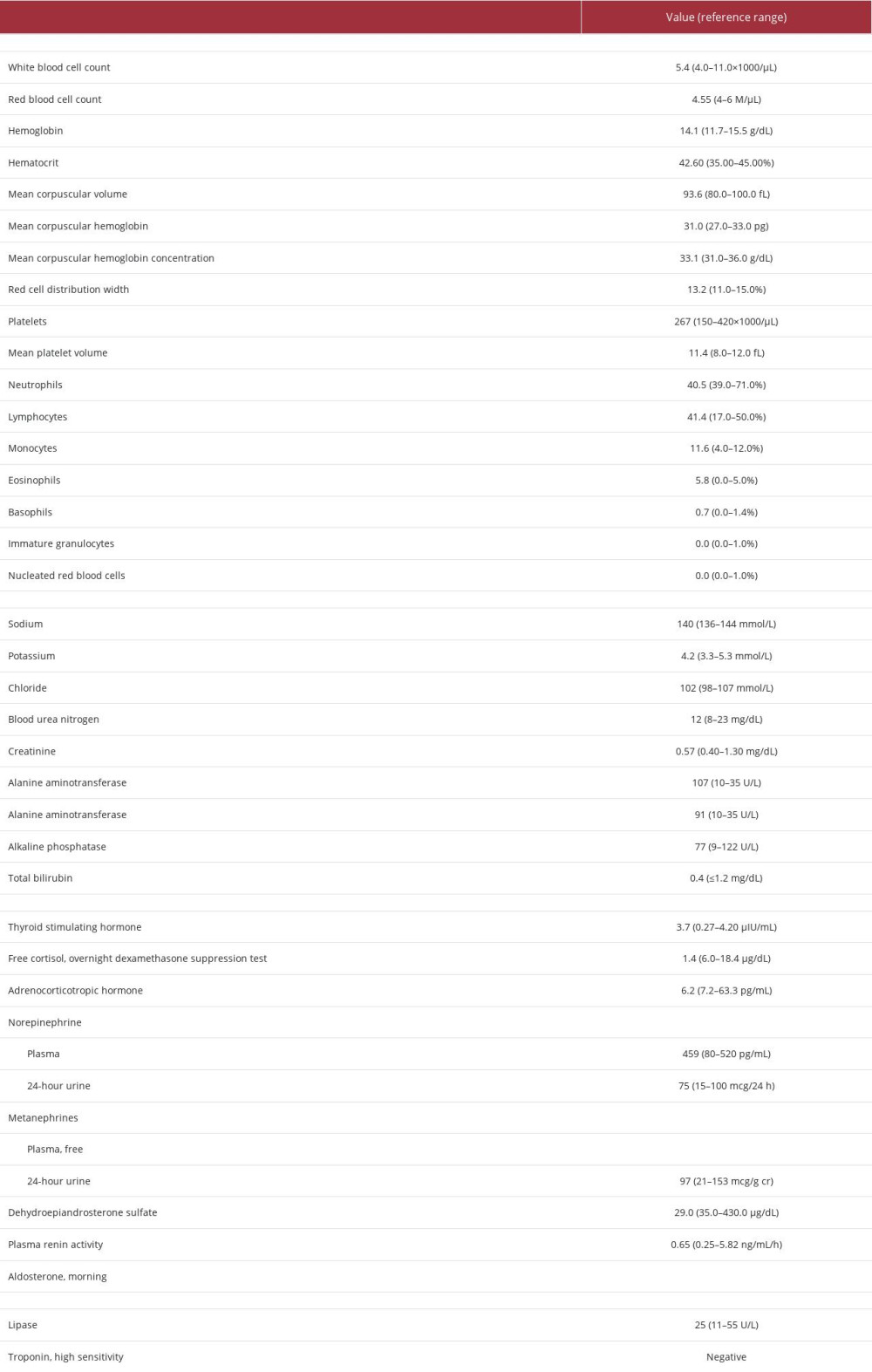 Table 1.. Case 1 laboratory findings upon presentation (initial workup).
Table 1.. Case 1 laboratory findings upon presentation (initial workup).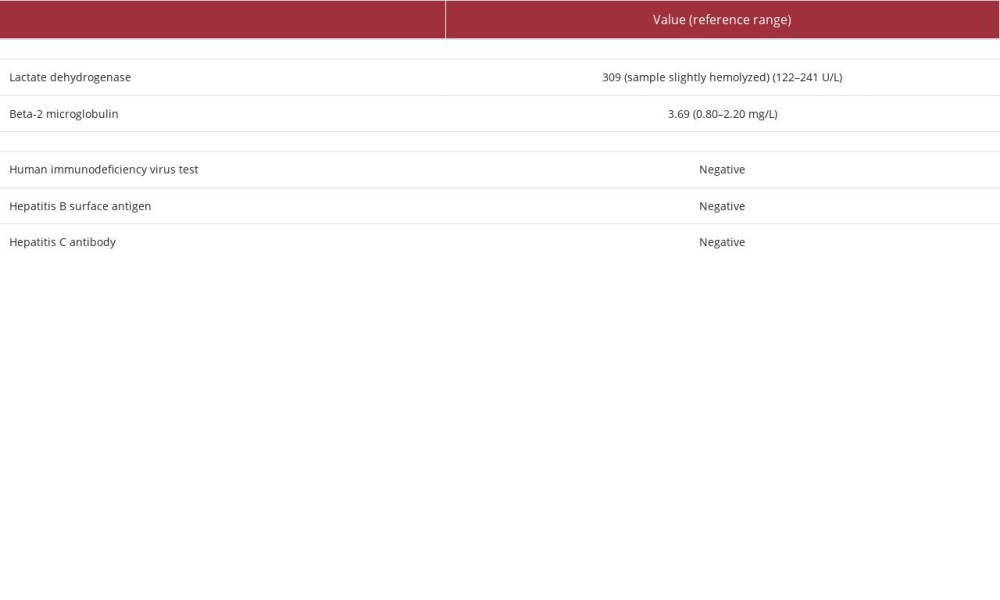 Table 2.. Case 1 post-tumor resection laboratory findings.
Table 2.. Case 1 post-tumor resection laboratory findings.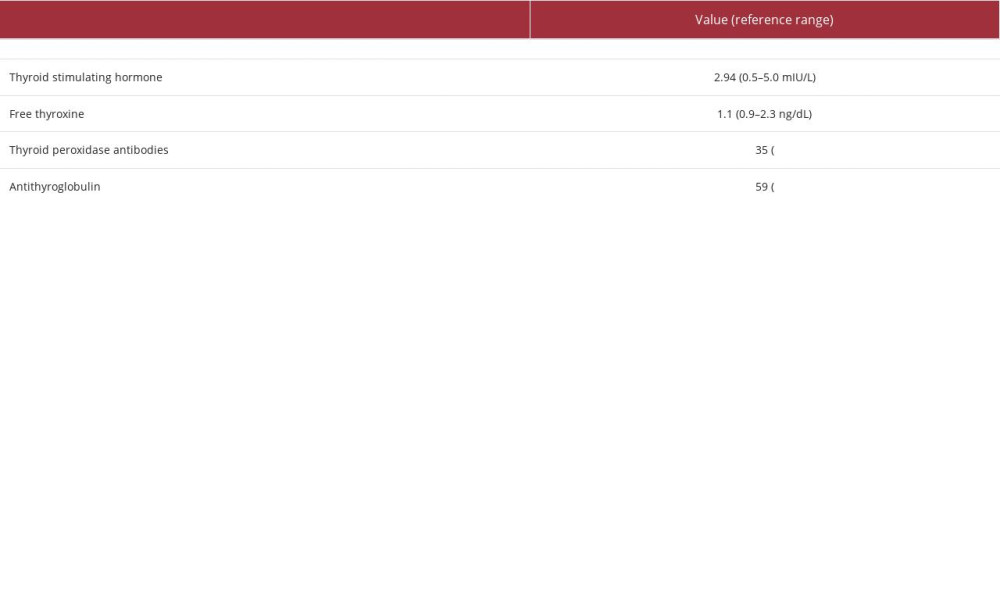 Table 3.. Case 2 thyroid tests upon presentation (initial workup).
Table 3.. Case 2 thyroid tests upon presentation (initial workup).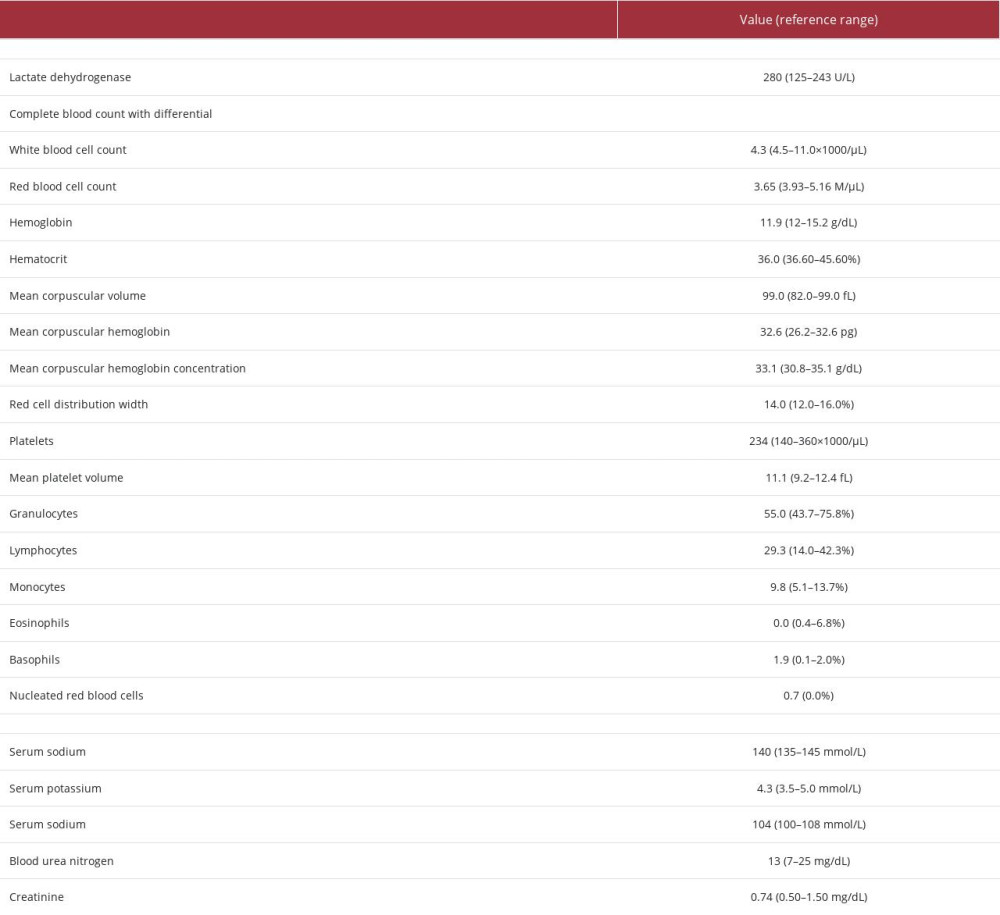 Table 4.. Case 2 post-tumor resection laboratory findings.
Table 4.. Case 2 post-tumor resection laboratory findings. Table 1.. Case 1 laboratory findings upon presentation (initial workup).
Table 1.. Case 1 laboratory findings upon presentation (initial workup). Table 2.. Case 1 post-tumor resection laboratory findings.
Table 2.. Case 1 post-tumor resection laboratory findings. Table 3.. Case 2 thyroid tests upon presentation (initial workup).
Table 3.. Case 2 thyroid tests upon presentation (initial workup). Table 4.. Case 2 post-tumor resection laboratory findings.
Table 4.. Case 2 post-tumor resection laboratory findings. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








