31 July 2020: Articles 
The Fetal Phenotype of Noonan Syndrome Caused by Severe, Cancer-Related Variants
Rare disease
Ieva Malniece1ABDE*, Adele Grasmane23BDEF, Inna Inashkina4BD, Janis Stavusis4BCE, Madara Kreile13AEF, Edvins Miklasevics5AG, Linda Gailite3DEGDOI: 10.12659/AJCR.922468
Am J Case Rep 2020; 21:e922468
Abstract
BACKGROUND: The nuchal translucency measurement is the major focus of an early fetal ultrasound scan, with the goal to identify various inherited conditions, such as chromosomal aberrations and others. The diagnostic strategy for fetuses with increased nuchal translucency and normal karyotype is not clearly defined and may vary between countries.
CASE REPORT: We describe 2 cases of Noonan syndrome diagnosed prenatally by ultrasound scanning and genetic testing. The prenatal ultrasound scans showed abnormal nuchal translucencies, cystic lymphangioma/cystic hygroma, and other findings. Both fetuses had normal karyotype; however, after additional analysis, pathogenic variants of the PTPN11 gene (encoding SH2 domain-containing protein tyrosine phosphatase) were found, previously frequently described as somatic variants in hematological malignancies in postnatal life, but not previously described with severe prenatal phenotype of Noonan syndrome.
CONCLUSIONS: Our case reports confirm the hypothesis that severe, cancer related PTPN11 variants cause severe Noonan syndrome prenatal phenotype, when inherited in the germline. Analysis of pathogenic variants associated with Noonan syndrome should be included in the prenatal diagnostics for fetuses with increased nuchal translucency and normal karyotype.
Keywords: Lymphangioma, Cystic, Noonan Syndrome, Nuchal Translucency Measurement, SH2 Domain-Containing Protein Tyrosine Phosphatases, Mutation, Pregnancy, Prenatal Diagnosis, Protein Tyrosine Phosphatase, Non-Receptor Type 11, Ultrasonography, Prenatal
Background
In approximately 3% of all pregnancies, fetal structural abnormalities can be visualized in an ultrasound scan, which can range from a single minor defect to severe and fatal multisystem anomalies [1].
Nuchal translucency (NT) is defined as the collection of fluid behind the neck of the fetus [2]. The definitions for increased NT vary in the literature, although any value ≥3.5 mm is ≥99th percentile for any gestational age between 11–13+6 weeks and is considered to be abnormal [2]. The causes of increased NT can vary greatly, chromosomal aneuploidies and aberrations being responsible for more than 50% of cases [3]. Therefore, NT measurement is the major focus of an early fetal scan to uncover possible inherited conditions [4–6]. Increased NT requires fetal karyotyping as well as detailed anatomic examination with fetal echocardiography in the second trimester [7]. In cases of increased NT and normal karyotype as well as chromosomal analysis results, the successive strategy is not clearly defined and may vary between countries and even hospitals.
In this report we describe the phenotype, genotype, and diagnostic strategies of 2 cases of Noonan syndrome, with increased NT and normal karyotype to emphasize the importance of prenatal diagnostics of Noonan syndrome and highlight the phenotypic features of severe, cancer-related
Case Reports
CASE 1:
A 37-year-old Caucasian female was referred to our department from the local health center due to an increased fetal nuchal translucency found during the first-trimester screening ultrasound scan. From anamnesis data, it was known that the patient had autosomal dominant polycystic kidney syndrome and 3 years prior she had had one normal delivery resulting in a healthy child. The patient did not have a history of smoking or of alcohol or substance abuse during pregnancy.
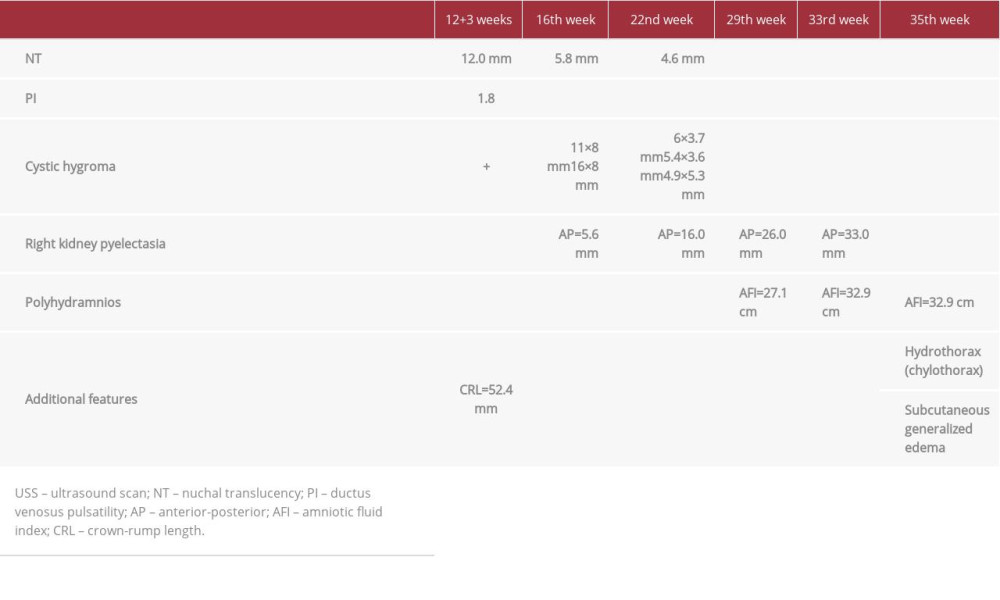
At the time of the fetal ultrasound scan, the fetus was 11 weeks and 1 day old with increased NT of 12.0 mm and increased ductus venosus pulsatility index of 1.8. On a detailed ultrasound scan, lymphatic dysgenesis with large multilocular fluid-filled cavities around the fetal neck was found and is shown in Figure 1. These findings were consistent with cystic lymphangioma/cystic hygroma. Chorionic villus sampling (CVS) was performed and the obtained material was referred for fluorescence in situ hybridization (FISH) testing, karyotyping, and PTPN11 gene (reference sequence: NG_007445911) mutation hotspot testing by Sanger sequencing.
During the follow-up visit, at the 16th week scan, the cystic hygroma was still present and the NT was significantly increased; in addition, kidney pyelectasis was diagnosed (Table 1). At this point the results of genetic testing were available, showing that the fetus had normal karyotype (46, XX), and no abnormalities were found by FISH (22q11.2). However, pathogenic de novo variant c.211T>C, p.Phe71Leu (rs397507512, Clinvar allele ID: 48969) of the PTPN11 gene was found, which confirmed the diagnosis of Noonan syndrome (shown in Figure 2). After a genetic consultation, the family decided to continue the pregnancy. An echocardiography scan was performed at the 22nd week, with no pathologic findings. During successive follow-up visits, we provided regular check-ups and ultrasound scans to evaluate the fetus. On the 35th week, during the last antenatal care visit, polyhydramnios and new phenotypic features were diagnosed, such as fetal hydrothorax (chylothorax) and subcutaneous generalized edema (nonimmune hydrops) (Table 1). At 35 weeks, a decision to perform partial amniotomy was made, because of polyhydramnios. After this procedure, partial placental abruption had started, therefore a cesarean section was performed. The newborn girl weighted 3080 g, with length of 46 cm and Apgar scores of 1 and 4 points at first and fifth minutes, respectively.
The newborn had a facial phenotype typical of Noonan syndrome: proptosis, epicanthal folds, ptosis, broad nasal bridge, hypertelorism, short neck, low-set ears, and abnormal auricles (similar to features seen in prenatal 3-dimensional ultrasound scan, Figure 3). The newborn had a lifespan of 25 days and passed away due to heart failure caused by ventricular and atrial septal defects, which had not been recognized prenatally.
CASE 2:
A 31-year-old Caucasian female presented to our department for the first-trimester screening of her second pregnancy. It was known that she was healthy, without chronic diseases or known risk factors such as smoking or alcohol abuse. Her first pregnancy was without complications and resulted in the delivery of a healthy child.
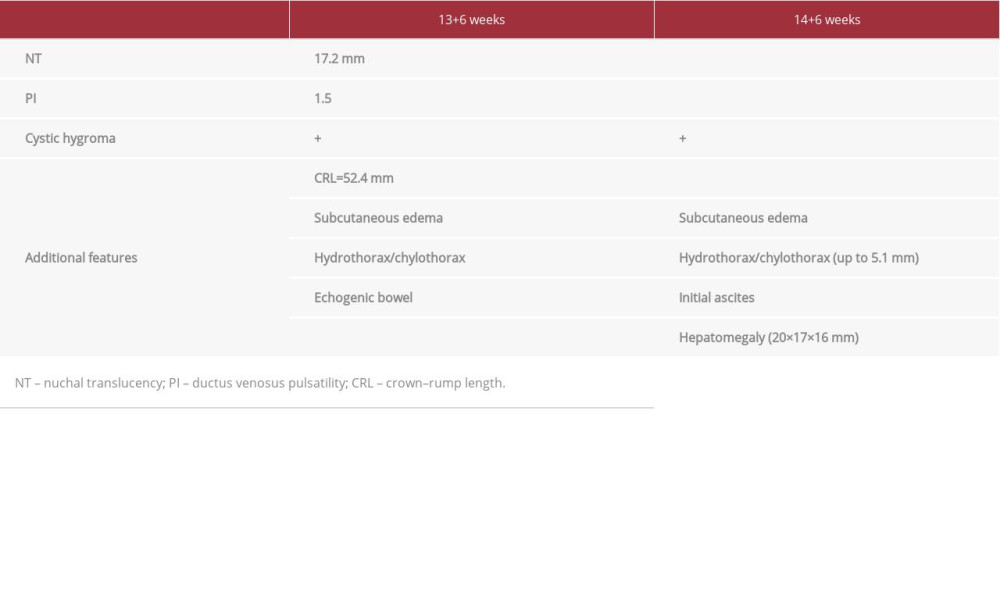
At the time of the fetal ultrasound scan, the fetus was 13 weeks and 6 days old, with increased NT of 17.2 mm and an increased ductus venosus pulsatility index (Table 2). Detailed ultrasound showed additional findings: cystic hygroma, sub-cutaneous edema, hydrothorax/chylothorax, and echogenic bowel (Figure 4, Table 2). Therefore, CVS was performed, and the obtained material was referred for genetic testing similar to what was described for Case 1.
During the follow-up ultrasound scan (14+6), subcutaneous edema and initial ascites were additionally diagnosed; furthermore, hydrothorax/chylothorax had increased, and hepatomegaly was suspected (Table 2).
Genetic testing showed that the fetus had a normal karyotype (46, XX) and no abnormalities were found by FISH (22q11.2), but the pathogenic de novo variant c.226G>C, p.Glu76Gln (rs121918464, ClinVar allele ID: 179445) of the PTPN11 gene was found, thus conforming the diagnosis of Noonan syndrome (Figure 2). After a genetic consultation, the family decided to terminate the pregnancy.
Discussion
This report presents 2 severe cases of Noonan syndrome that were diagnosed prenatally, presenting with increased NT in an ultrasound scan. Furthermore, it emphasizes the importance of Noonan syndrome testing in prenatal settings, as well as highlights the phenotype-genotype link in severe Noonan syndrome cases.
Prenatal screening with ultrasound allows the evaluation of gross fetal abnormalities and nuchal translucency thickness [8]. At present, NT measurement is primarily used to detect chromosomal aneuploidies [8], although there are many reasons for increased NT, including various genetic syndromes, cardiac anomalies, and other structural anomalies [9].
Cystic hygroma is associated with extremely increased NT. In approximately 50% of cases with cystic hygroma, it is caused by a chromosomal aneuploidy, while 30% of cases have an additional structural anomaly associated with other conditions, and 20% of fetuses develop normally [4]. Importantly, testing for Noonan syndrome is not currently included in guidelines for prenatal testing [4–6], although it could additionally solve up to 20% of cases with increased NT and even up to 30% in cases with cystic hygroma [10].
Some published studies suggest that Noonan syndrome can be suspected prenatally in cases with large NT in addition to one or more of the following characteristics: cystic hygroma, pleural effusion, hydrops fetalis, cardiac anomalies, or specific facial features [11].
Noonan syndrome is a genetically heterogeneous and pathogenic variants of more than 10 genes are known to be implicated in its development [12]. Approximately 50% of Noonan syndrome cases have a pathogenic variant of the
In Latvia, we have implemented the strategy of performing karyotype and FISH analysis for the most common aberrations in case of increased NT. Euploid fetuses are also tested for Noonan syndrome by Sanger sequencing for mutation hotspots in the genes most commonly involved in Noonan syndrome –
The SHP2 is a Src homology 2 (SH2) domain-containing protein-tyrosine phosphatase that positively modulates Ras function. Ras proteins are known to be signaling molecules that regulate a variety of cellular processes, including cell growth, differentiation, the mitotic cycle, and oncogenic transformation [14]. It is interesting to note that both
Our report emphasizes the role of testing for Noonan syndrome in cases with increased NT/cystic hygroma and suggests testing primarily for mutation hotspots of Noonan syndrome
Conclusions
Our case reports confirm the hypothesis that severe, cancer related
Figures
References:
1.. Lord J, McMullan DJ, Eberhardt RY, Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study: Lancet, 2019; 393(10173); 747-57
2.. Shakoor S, Dileep D, Tirmizi S, Increased nuchal translucency and adverse pregnancy outcomes: J Matern Fetal Neonatal Med, 2017; 30(14); 1760-63
3.. Nicolaides KH, Brizot ML, Snijders RJ, Fetal nuchal translucency: Ultrasound screening for fetal trisomy in the first trimester of pregnancy: Br J Obstet Gynaecol, 1994; 101(9); 782-86
4.. , Practice Bulletin No. 163: Screening for fetal aneuploidy: Obstet Gynecol, 2016; 127(5); e123-37
5.. , Committee Opinion No. 682: Microarrays and next-generation sequencing technology: The use of advanced genetic diagnostic tools in obstetrics and gynecology: Obstet Gynecol, 2016; 128(6); e262-68
6.. Grande M, Jansen FAR, Blumenfeld YJ, Genomic microarray in fetuses with increased nuchal translucency and normal karyotype: A systematic review and meta-analysis: Ultrasound Obstet Gynecol, 2015; 46(6); 650-58
7.. Sahin Uysal N, Gulumser C, Yilmaz Celik Z, Yanik FB, Increased nuchal translucency and pregnancy outcomes: Experience of Baskent University Ankara Hospital: Turkish J Obstet Gynecol, 2019; 16(2); 100-6
8.. Salomon LJ, Alfirevic Z, Bilardo CM, ISUOG practice guidelines: Performance of first-trimester fetal ultrasound scan: Ultrasound Obstet Gynecol, 2013; 41(1); 102-13
9.. Socolov D, Socolov R, Gorduza VE, Increased nuchal translucency in fetuses with a normal karyotype-diagnosis and management: An observational study: Medicine (Baltimore), 2017; 96(29); e7521
10.. Croonen EA, Nillesen WM, Stuurman KE, Prenatal diagnostic testing of the Noonan syndrome genes in fetuses with abnormal ultrasound findings: Eur J Hum Genet, 2013; 21(9); 936-42
11.. Bakker M, Pajkrt E, Bilardo CM, Increased nuchal translucency with normal karyotype and anomaly scan: What next?: Best Pract Res Clin Obstet Gynaecol, 2014; 28(3); 355-66
12.. Grant AR, Cushman BJ, Cave H, Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework: Hum Mutat, 2018; 39(11); 1485-93
13.. El Bouchikhi I, Belhassan K, Moufid FZ, Noonan syndrome-causing genes: Molecular update and an assessment of the mutation rate: Int J Pediatr Adolesc Med, 2016; 3(4); 133-42
14.. Li S, Hsu DD, Wang H, Feng G-S, Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis: Front Med, 2012; 6(3); 275-79
15.. Chang MT, Bhattarai TS, Schram AM, Accelerating discovery of functional mutant alleles in cancer: Cancer Discov, 2018; 8(2); 174-83
16.. Tartaglia M, Martinelli S, Stella L: Am J Hum Genet, 2006; 78(2); 279-90
17.. Mason-Suares H, Toledo D, Gekas J, Juvenile myelomonocytic leukemia-associated variants are associated with neo-natal lethal Noonan syndrome: Eur J Hum Genet, 2017; 25(4); 509-11
18.. Tate JG, Bamford S, Jubb HC, COSMIC: The catalogue of somatic mutations in cancer: Nucleic Acids Res, 2019; 47(D1); D941-47
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250