16 September 2020: Articles 
Tumor Lysis Syndrome After a Single Dose of Atezolizumab with Nab-Paclitaxel: A Case Report and Review of Literature
Unusual or unexpected effect of treatment
Xavier Carrier1EF, Sumit Gaur2E, Alexander Philipovskiy2ADEF*DOI: 10.12659/AJCR.925248
Am J Case Rep 2020; 21:e925248
Abstract
BACKGROUND: Tumor lysis syndrome (TLS) represents a severe and dangerous side effect of chemotherapy. The frequency of TLS is not well known in patients with breast cancer, and there are no reports of TLS after the second or third lines of chemotherapy or immunotherapy combined with chemotherapy in these patients.
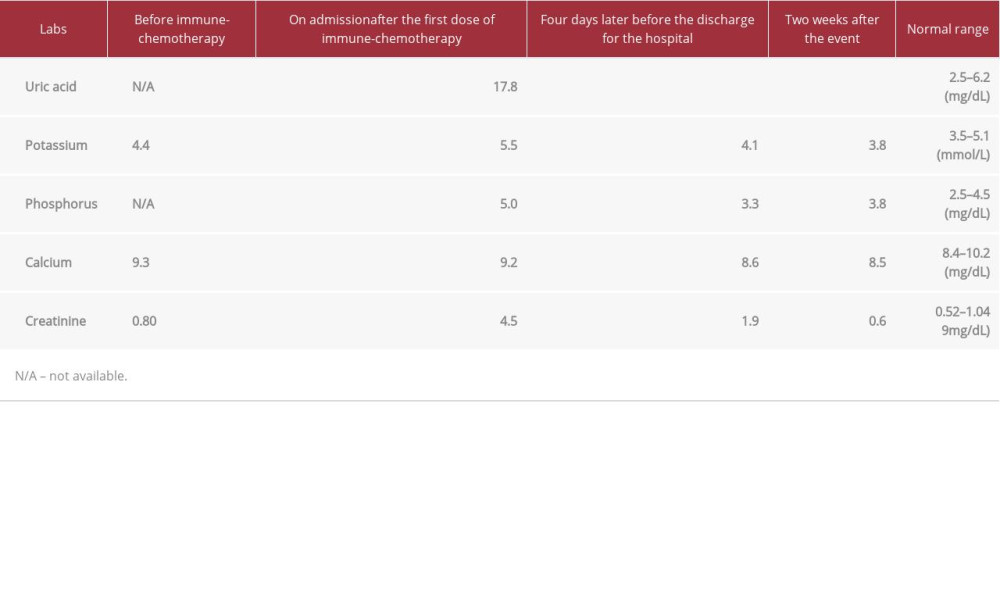
CASE REPORT: We present the case of a 55-year-old postmenopausal woman with metastatic triple-negative breast cancer who received multiple lines of chemotherapy and developed TLS after receiving combined chemoimmunotherapy. She presented to our medical center with generalized body weakness, sleepiness, anorexia, and oliguria 6 days after her first dose of combined chemoimmunotherapy with nanoparticle albumin–bound (nab)-paclitaxel (100 mg/m²) and atezolizumab (840 mg). A complete blood count on admission showed pancytopenia, with serum levels of uric acid at 17.8 mg/dL, creatinine at 3.4 mg/dL, potassium at 5.5 mEq/L, phosphorus at 5.0 mg/dL, and calcium at 9.3 mg/dL. TLS (grade 2) was diagnosed based on reported Cario-Bishop criteria, and the patient was promptly treated with intravenous hydration and a single dose of rasburicase (0.15 mg/kg). Symptoms completely resolved within 4 days, and the patient was discharged home.
CONCLUSIONS: We present a case of TLS after combined therapy with atezolizumab and nab-paclitaxel in a heavily pretreated patient with metastatic triple-negative breast cancer. Medical oncologists and general practice clinicians need to be aware of the possibility of TLS, even in unlikely cases, and to recognize the clinical signs of TLS to enable prompt and appropriate management.
Keywords: Immunotherapy, Active, Triple Negative Breast Neoplasms, Tumor Lysis Syndrome, Albumins, Antibodies, Monoclonal, Humanized, paclitaxel
Background
Tumor lysis syndrome (TLS) is a severe adverse effect of chemo-therapy, whereby extensive lysis of cancer cells causes electrolyte abnormalities and multiorgan failure [1]. The electrolyte abnormalities in TLS can cause a constellation of symptoms leading to multiorgan failure and death. TLS is more common in hematologic malignancies and has rarely been reported in nonhematologic solid tumors. Some data suggest that the incidence of TLS can be as high as 31% in acute leukemia, aggressive non-Hodgkin lymphoma, and Burkitt leukemia/lymphoma [2,3]. The incidence is even higher among children with acute lymphoblastic leukemia, approaching 45% [4]. In contrast, TLS is sporadic in solid tumors, with only case reports having been presented [5–7]. The incidence of TLS in breast cancer is not well known, and there are no reports of TLS after the second or third lines of chemotherapy or after immunotherapy.
Case Report
A 55-year-old Hispanic woman with metastatic triple-negative breast cancer (mTNBC) with extensive liver and bone metastasis came to the emergency department of our medical center in January 2020. She reported generalized body weakness, sleepiness, anorexia, and oliguria for the past few days. A complete blood count on admission showed pancytopenia and serum levels of uric acid at 17.8 mg/dL, creatinine at 3.4 mg/dL, potassium at 5.5 mEq/L, phosphorus at 5.0 mg/dL, and calcium at 9.3 mg/dL. She had received her first cycle of atezolizumab and nanoparticle albumin–bound (nab)-paclitaxel 6 days before admission. Her medical history was significant for breast cancer, which was initially diagnosed in 2014 as stage IIIA (T3N2) invasive ductal carcinoma of the left breast. Hormonal status was estrogen receptor (ER) positive (60%), progesterone receptor (PR) positive (30%), and HER2/neu negative. The patient underwent wide local excision with axillary lymph node biopsy followed by 4 cycles of adjuvant chemo-therapy (dense dose doxorubicin and cyclophosphamide) followed by 12 cycles of weekly Taxol. She completed 31 days of adjuvant radiation therapy in 2015. She started anti-estrogen therapy with exemestane at the same time as adjuvant radiation therapy. In October 2017, she was found to have multiple liver lesions. A liver biopsy showed high-grade metastatic invasive ductal carcinoma ER negative, PR negative, and Her2-neu negative (mTNBC). The tumor mutation profile was requested from Foundation Medicine and showed the following mutations: EGFR (amplification), PIK3CA (H1047R), MYC (amplification), RAD21 (amplification), RAD51 (BQ23), and TP53 (C275Y). The tumor proportion score was 0% (Daco 22C3). The patient received 3 cycles of palliative chemotherapy with gemcitabine (1000 mg/m2) and carboplatin (5 AUC) [8] and initially showed stable disease based on RACIS 1.1 criteria [9]. However, markers rose very soon afterward, and disease progression was suspected. The patient’s chemotherapy was changed to the next line with eribulin (1.4 mg/m2) [10].
Interestingly, her tumor was remarkably sensitive to eribulin, and her imaging study showed a complete response based on RACIS 1.1. The patient decided to continue with chemotherapy until disease progression because her quality of life was not affected by chemotherapy, and she experienced only grade 1 peripheral neuropathy. After completion of 15 cycles of eribulin (up to August 2019), her disease was documented as stable. She decided to stop her treatment at that time. In November 2019, she presented to our hospital with a new onset of back pain. Magnetic resonance imaging of the spine showed multiple metastatic bone lesions and pathological fracture of the L3 vertebra. The patient underwent kyphoplasty and received local palliative radiation therapy. The computerized tomography scan of the chest, abdomen, and pelvis showed multiple new lesions in the liver and lung. The bone biopsy was positive for metastatic triple-negative adenocarcinoma. The patient received combined immunochemotherapy with anti-PD-L1 agent atezolizumab 840 (mg/m2) and nab-paclitaxel (100 mg/m2) [11], with the first cycle administered 6 days before her hospital admission. She was admitted to our hospital and TLS was subsequently diagnosed in January 2020. The patient underwent aggressive intravenous hydration and a dose of rasburicase (6 mg). She experienced significant clinical improvement with complete resolution of TLS symptoms, and she was discharged 4 days after admission (Table 1).
Discussion
Breast cancer represents one of the most common types of cancer in women in the United States. It is the second most common cause of cancer death after lung cancer [12,13]. Triple-negative breast cancer constitutes 15% to 20% of all breast cancer. It is biologically defined by the absence of receptors for estrogen, progesterone, and human epidermal growth factor, and it is associated with poor outcomes [14]. Systemic chemotherapy has been the only treatment option for these patients for decades [15].
Interestingly, mTNBC is initially sensitive to chemotherapy, with a response rate of up to 60% [16]. However, high response rates do not translate to survival outcomes. Typical progression-free survival after the first line of chemotherapy can vary from 5 to 8 months. After the first progression, the disease typically becomes resistant to subsequent lines of chemotherapy, leading to death within the next 3 to 5 months [14,15]. Modern advances in immunotherapy have shifted the treatment approach for multiple solid tumors. Immunotherapy is now the first-line treatment for metastatic melanoma, renal cell carcinoma, and lung cancer [17–19]. In March 2019, a new combination therapy composed of the anti-PD-L1 antibody atezolizumab and protein-bound paclitaxel received full Food and Drug Administration approval for patients with mTNBC. A study of its efficacy (IMpassion 130) showed significantly improved disease-free and overall survival, particularly in patients with upregulated PD-L1 expression [11].
The exact mechanism of the anti-PD-L1 blockade is unclear. One of the most accepted theories suggests that antitumor T cells are generally capable of recognizing tumor cells through detection of abnormal peptides associated with MHC class I molecules. PD-1 is not expressed on resting T cells, but it is inducible and expressed after activation by TCR/MHC and CD28/B7 interactions in response to a foreign antigen or mutated (cancer) antigen. Cancer cells protect themselves from the immune recognition and destruction by expressing PD-L1 (ligand). When engaged by its ligands, the PD-1 axis dampens T-cell responses [20]. Atezolizumab selectively binds to PD-L1, which prevents interaction with PD-1 and B7-1 and reverses T-cell suppression. Consequently, T cells are able to target the tumor cells and physically destroy them. Massive destruction of tumor cells may explain the occurrence of TLS.
Typically, TLS can be diagnosed clinically based on specific abnormal laboratory values. The modern definition of TLS was proposed by Cairo and Bishop [1]. Our patient developed TLS 6 days after receiving combined immunochemotherapy, and she met 3 of 4 positive criteria for TLS (elevated uric acid, potassium, and phosphorus). The severity of the TLS was grade 2 (based on creatinine level).
The literature contains only a minimal number of TLS cases in patients with breast cancer after conventional chemotherapy, with no reports on TLS after immunotherapy or combined chemoimmunotherapy [6,21–24]. In our case, TLS developed after the first dose of combined chemoimmunotherapy. TLS is well documented to be more common in highly proliferating malignancies, such as high-grade lymphomas or acute leukemias [3]. In contrast, it is rare in solid tumors. The reasons for this phenomenon are presently unclear. The frequency of TLS in invasive breast carcinoma unknown, but it has been reported in a few cases, most often after the first dose of chemo-therapy in so-called chemotherapy-naïve patients [7,21,22].
In our case, we believe that TLS was purely due to immuno-therapy because our patient had previously received multiple lines of chemotherapy without any evidence of subsequent TLS. The fact that her tumor proportion score was 0% from her biopsy in October 2017 might be explained by inconsistency and/or high variation of PD-L1 staining techniques, which was previously described in the literature [25]. Because the patient received multiple lines of chemotherapy after her biopsy and was in prolonged remission, the PD-L1 might have changed. In addition, chemotherapy itself can upregulate the expression of PD-1 [26].
A review article by Howard et al. [27] describes key features associated with a higher possibility of TLS. Our patient had such risk factors, including extensive metastasis and organ infiltration. Further, a few cases have been reported over the years describing TLS in breast cancer. One such case was a 52-year-old woman with metastatic, recurrent ductal cell carcinoma of the breast, who presented with TLS after a single dose of paclitaxel [21]. Aslam et al. [22] described a chemotherapynaïve 58-year-old woman who developed TLS after the first dose of gemcitabine, while Baudon et al. [23] reported TLS in another chemotherapy-naïve 58-year-old woman after a single dose of trastuzumab and pertuzumab.
Rare cases of TLS have been described in patients who received immunotherapy. Fa’ak et al. [6] reported a case in which TLS occurred after the initial dose of atezolizumab for metastatic urothelial cancer [6]. In a search of PubMed, we were able to identify only 4 case reports of TLS from 2014 to 2018 after immunotherapy for solid tumors. Two cases reported TLS after a single dose of nivolumab, and 2 after atezolizumab [28–31]. No reports have been published for triple-negative breast cancer and chemotherapy combined with immunotherapy.
Conclusions
We have presented the first case report of TLS diagnosed after a single dose of atezolizumab and nab-paclitaxel therapy. TLS is rare in solid tumors, and very few cases have been reported for chemotherapy and for immunotherapy. The Food and Drug Administration only recently approved treatment with atezolizumab and nab-paclitaxel for mTNBC, and the recency of that approval, along with the rarity of TLS in solid tumors, likely explains why no other cases of TLS have been reported. It is essential to recognize the clinical signs of TLS, even in unlikely cases, and to know the appropriate management.
References:
1.. Cairo MS, Bishop M, Tumour lysis syndrome: New therapeutic strategies and classification: Br J Haematol, 2004; 127; 3-11
2.. Darmon M, Vincent F, Camous L, Tumour lysis syndrome and acute kidney injury in high-risk haematology patients in the rasburicase era. A prospective multicentre study from the Groupe de Recherche en Reanimation Respiratoire et Onco-Hematologique: Br J Haematol, 2013; 162; 489-97
3.. Belay Y, Yirdaw K, Enawgaw B, Tumor lysis syndrome in patients with hematological malignancies: J Oncol, 2017; 2017; 9684909
4.. Coiffier B, Altman A, Pui CH, Guidelines for the management of pediatric and adult tumor lysis syndrome: An evidence-based review: J Clin Oncol, 2008; 26; 2767-78
5.. Elinoff JM, Salit RB, Ackerman HC, The tumor lysis syndrome: N Engl J Med, 2011; 365; 571-74
6.. Fa’ak F, Vanegas D, Osei KM, A case report of atezolizumab induced tumor lysis syndrome: Am J Case Rep, 2019; 20; 785-89
7.. Mirrakhimov AE, Ali AM, Khan M, Barbaryan A, Tumor lysis syndrome in solid tumors: An up to date review of the literature: Rare Tumors, 2014; 6; 5389
8.. O’Shaughnessy J, Schwartzberg L, Danso MA, Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer: J Clin Oncol, 2014; 32; 3840-47
9.. Schwartz LH, Litiere S, de Vries E, RECIST 1.1-Update and clarification: From the RECIST committee: Eur J Cancer, 2016; 62; 132-37
10.. Cortes J, O’Shaughnessy J, Loesch D, Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study: Lancet, 2011; 377; 914-23
11.. Schmid P, Adams S, Rugo HS, Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: N Engl J Med, 2018; 379; 2108-21
12.. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69; 7-34
13.. DeSantis CE, Ma J, Gaudet MM, Breast cancer statistics, 2019: Cancer J Clin, 2019; 69; 438-51
14.. Philipovskiy A, Corral J, Dwivedi KA, Efficacy of neoadjuvant versus adjuvant chemotherapy in Hispanic/Latino (H/L) women with local or locally advanced triple-negative breast cancer (TNBC): In Vivo, 2019; 33; 1227-34
15.. Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T, Metastatic and triple-negative breast cancer: challenges and treatment options: Drug Deliv Transl Res, 2018; 8; 1483-507
16.. Schmid P, Cortes J, Pusztai L, Pembrolizumab for early triple-negative breast cancer: N Engl J Med, 2020; 382; 810-21
17.. Petrova V, Arkhypov I, Weber R, Modern aspects of immunotherapy with checkpoint inhibitors in melanoma: Int J Mol Sci, 2020; 21(7); 2367
18.. Cho YH, Kim MS, Chung HS, Hwang EC, Novel immunotherapy in metastatic renal cell carcinoma: Investig Clin Urol, 2017; 58; 220-27
19.. Shroff GS, de Groot PM, Papadimitrakopoulou VA, Targeted therapy and immunotherapy in the treatment of non-small cell lung cancer: Radiol Clin North Am, 2018; 56; 485-95
20.. Akinleye A, Rasool Z, Immune checkpoint inhibitors of PD-L1 as cancer therapeutics: J Hematol Oncol, 2019; 12; 92
21.. Vaidya GN, Acevedo R, Tumor lysis syndrome in metastatic breast cancer after a single dose of paclitaxel: Am J Emerg Med, 2015; 33(2); 308.e1-2
22.. Aslam HM, Zhi C, Wallach SL, Tumor lysis syndrome: A rare complication of chemotherapy for metastatic breast cancer: Cureus, 2019; 11; e4024
23.. Baudon C, Duhoux FP, Sinapi I, Canon JL, Tumor lysis syndrome following trastuzumab and pertuzumab for metastatic breast cancer: A case report: J Med Case Rep, 2016; 10; 178
24.. Guo L, Song P, Xue X, Variation of programmed death ligand 1 expression after platinum-based neoadjuvant chemotherapy in lung cancer: J Immunother, 2019; 42; 215-20
25.. Kluger HM, Zito CR, Turcu G, PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors: Clin Cancer Res, 2017; 23; 4270-79
26.. Ng HY, Li J, Tao L, Chemotherapeutic treatments increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK activation: Transl Oncol, 2018; 11; 1323-33
27.. Howard SC, Jones DP, Pui CH, The tumor lysis syndrome: N Engl J Med, 2011; 364; 1844-54
28.. Brunnhoelzl D, Wang J, Acute tumor lysis syndrome after anti-pd-1 immuno-therapy nivolumab for metastatic melanoma: J Mol Oncol Res, 2017; 1(1); 5-6
29.. Sater HA, Patel RM, Sullivan BT, Parikh J, A case report of inflammatory syndrome presenting as tumor lysis syndrome after single dose of nivolumab: J Cancer Prev Curr Res, 2017; 8(2); 00271
30.. Brunnhoelzl D, Weed M, Trepet R, Wang J, Tumor lysis syndrome following a single atezolizumab infusion for metastatic urothelial carcinoma involving both upper and lower tract: Arch Cancer Res, 2017; 5(1); 127
31.. Herbst RS, Soria JC, Kowanetz M, Predictive correlates of response to anti-PD-L1 antibody MPDL3280A in cancer patients: Nature, 2014; 515(7528); 563-67
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250