19 October 2020: Articles 
A Cecal Mucormycosis Mass Mimicking Colon Cancer in a Patient with Renal Transplant: A Case Report and Literature Review
Unusual clinical course, Challenging differential diagnosis, Management of emergency care
Saleh Busbait1ABCDEF*, Zainab AlMusa2BDF, Mohammed Al Duhileb3AB, Ayed A. Algarni4BCD, Ameera Balhareth3ACDFDOI: 10.12659/AJCR.926325
Am J Case Rep 2020; 21:e926325
Abstract
BACKGROUND: Mucormycosis is a rare, invasive, and opportunistic fungal infection that occurs in the setting of neutropenia, immune deficiency, solid-organ transplant, and iron overload. The gastrointestinal system is a rare site of mucormycosis, and gastrointestinal mucormycosis is associated with high mortality and accounts for 4–7% of all cases.
CASE REPORT: We present the case of a 64-year-old hypertensive man with transfusion-dependent myelodysplastic syndrome who underwent renal transplant surgery 11 years ago. He also was taking maintenance Deferasirox for iron overload. He presented with a 2-day history of right lower-quadrant abdominal pain, nausea, vomiting, and non-bloody diarrhea. An abdominal examination revealed guarding and a 5×6 cm mass in the right iliac fossa. A CT scan of the abdomen showed signs of perforation of a cecal mass. As the patient was unstable, emergency right hemicolectomy and end ileostomy were performed. After the surgery, the patient was moved to the Intensive Care Unit (ICU) and a broad-spectrum antibiotic was administered. Histopathological examination results received on postoperative day 5 showed broad pauciseptate hyphae with substantial blood-vessel infiltration, suggestive of mucormycosis. Amphotericin B was started; however, on the same day, his condition deteriorated and he was moved back to the ICU. Despite maximum cardiorespiratory support, he had multiorgan failure and died.
CONCLUSIONS: Gastrointestinal mucormycosis presentation is non-specific, the diagnosis is often made late or is missed, and mortality remains high. High clinical suspicion, early diagnosis, and combined antifungal and surgical treatment is the best way to reduce mortality and improve survival.
Keywords: Immunosuppression, Iron Overload, Kidney Transplantation, mucormycosis, Myelodysplastic syndromes, Zygomycosis, amphotericin B, Antifungal Agents, Colonic Neoplasms
Background
Mucormycosis is a rare but highly invasive opportunistic fungal infection that occurs in renal transplant recipients. Neutropenia, immune deficiencies, malignant disease, malnutrition, diabetes, trauma, organ transplantation, and iron overload are some of the risk factors reported in the literature [1]. Clinical infection with fungi of the order Mucorales causes rhinocerebral, pulmonary, cutaneous, gastrointestinal, and disseminated diseases [1].
Gastrointestinal mucormycosis is rare and accounts for 4–7% of all mucormycoses, and the colon is involved in 32% of all cases of gastrointestinal mucormycosis [2]. In patients who received a solid-organ transplant, the colon is only involved in 7.6% of gastrointestinal mucormycosis [3]. In this report, we present a case of colonic mucormycosis that mimicked colon cancer in a renal transplant recipient.
Case Report
The patient was a 64-year-old hypertensive man with transfusion-dependent myelodysplastic syndrome (MDS) and chronic kidney disease who underwent renal transplant surgery 11 years ago and was maintained on tacrolimus with stable graft function. He was diagnosed with MDS more than 1 year ago, had received 8 cycles of azacitidine, and the last cycle was received 5 months ago. While on azacitidine, he received multiple doses of Filgrastim G-CSF for neutropenia and was on prophylactic antifungal (fluconazole). Prophylactic fluconazole was stopped 4 months before presentation as he was not neutropenic at that time. He also had iron overload; his last ferritin level >10 000 ng/ml (normal range, 21–274 ng/ml) and he was on maintenance Deferasirox.
He presented to the Emergency Department with a 2-day history of right lower-quadrant abdominal pain, nausea, and bilious vomiting associated with non-bloody diarrhea. He did not have a fever but had unintentional weight loss of more than 10 kg in the last 6 months. He denied any bowel habit changes or other gastrointestinal symptoms in recent months. On physical examination, the patient was found to be in pain. His vital signs were as follows: blood pressure of 104/62 mmHg, pulse of 105 bpm, temperature of 37.2°C, and oxygen saturation of 98% on 2 L oxygen nasal cannula. Abdominal examination revealed guarding, a 5×6 mass in the right iliac fossa with mild tenderness over the palpable mass but no rebound tenderness, and sluggish bowel sounds. A digital rectal examination revealed normal rectal tone and normal stool on the examining finger but no mass or blood.
Blood analysis showed a white blood cell count of 4.3×109/L (normal range, 4.5–11×109/L), neutrophil count was 1650/mm3 (normal range, 2500-7000/mm3), hemoglobin concentration of 7.7 g/dL (normal range, 13.5–17.5 g/dL), platelet count of 119×109/L (normal range, 150–400×109/L), blood urea nitrogen concentration of 13.4 mmol/L (normal range, 2.7–7.2 mmol/L), creatinine level of 187 μmol/L (normal range, 62–115 μmol/L), sodium ion concentration of 132 mmol/L (normal range, 135–147 mmol/L), potassium ion concentration of 4.5 mmol/L (normal range, 3.5–5.1 mmol/L), and a lactate concentration of 1.5 mmol/L (normal range, 0.4–2.0 mmol/L). A blood culture showed no growth, and urinalysis and culture were negative.
We then performed abdominal computed tomography (CT) without contrast due to his deranged renal function, and it revealed a 3-cm circumferential wall thickening of an 8-cm length of cecum, which resulted in luminal narrowing with ulcerations and air along the thickened wall that extended to the right lateral abdominal wall and possibly concealed a perforation. There was significant surrounding fat stranding, which had a thickness of 1.2 cm, and small lymph nodes in close proximity. There was no evidence of bowel obstruction, and this made us consider the presence of a concealed perforated cecal mass (Figures 1, 2). Because colon cancer has a higher incidence and is more aggressive in renal transplant patients in our patient’s age range, perforated colonic malignancy was our working diagnosis at that time.
The condition of our patient then started to become unstable, with tachycardia and hypotension (septic shock), and he was administered intravenous fluid and broad-spectrum antibiotics (piperacillin-tazobactam). Laparotomy revealed turbid fluid in the abdomen and a 9×5×4 cm cecal mass with no obvious perforation and no necrotic tissue, and no debridement was done, giving us the impression of cecal malignancy with impending perforation. The patient’s condition was unstable during the operation; therefore, right hemicolectomy with end ileostomy and distal mucous fistula was performed.
After the surgery, the patient was moved to the ICU, where he was intubated without inotropic support, and his antibiotic treatment was changed from piperacillin-tazobactam to imipenem. Fluconazole was added prophylactically to cover for invasive candida as he was immunocompromised and critically ill. His nasogastric tube output was bloody, and he was managed conservatively with pantoprazole infusion and blood products. His condition slowly improved. He was extubated on postoperative day 3, and moved from the ICU on postoperative day 4. We were informed the next day by the pathologist that periodic acid-Schiff staining showed the colon mass heavily infiltered with broad pauciseptate hyphae (Figure 3) with evidence of thrombosis and angioinvasion of the vascular wall by hyphae (Figure 4), which is consistent with mucormycosis. At that time, we realized that we had been misled by the patient’s risk factors of malignancy, and in fact he had a fungal mass mimicking a malignancy. Fluconazole was replaced with amphotericin B, with his latest Cr 223 μmol/L (normal range, 62–115 μmol/L), knowing it would worsen his kidney function. The next day, his condition deteriorated as he became hypotensive, and he was moved back to the ICU and intubated with inotropic support. An abdominal CT did not show any signs of bowel ischemia or perforation. However, the patient developed septic shock and multiorgan failure and died.
Discussion
Mucormycosis is a rare opportunistic infection caused by a fungus of the class Zygomycetes [1]. The morphology of the hyphae is the main feature that differentiates Zygomycetes from other hyaline filamentous fungi. The morphological features of Zygomycetes include wide coenocytic ribbon-like hyphae with wide-angle branching [4]. Zygomycetes are often described by surgical pathologists and in the literature as aseptate; however, pauciseptate is a more accurate description, as the septae occur at irregular intervals. It is important to use the correct descriptive term to avoid mistaking the fungus for septate fungi such as Aspergillus [5].
Colonic mucormycosis can present with relatively less severe symptoms such as fever, abdominal pain, distension, and diarrhea [6]. It can also present more severely with gastrointestinal bleeding and visceral perforation, which causes peritonitis or mimics gastrointestinal malignancy, as in our patient [6]. The most common presenting symptoms of gastrointestinal mucormycosis are abdominal pain (68%), gastrointestinal bleeding (48%), and fever with change in bowel habit (<20%) [7]. Gastrointestinal mucormycosis is rare and accounts for 4–7% of all mucormycoses, and there is colon involvement in 32% of patients [2]. In a review of 87 patients with gastrointestinal mucormycosis, the most commonly affected organs were the stomach (57.5%), colon (32.2%), small bowel (10.4%), and esophagus (7%) [8].
Mucormycosis is an invasive opportunistic fungal infection that occurs in patient with neutropenia, immune deficiencies, malignant disease, malnutrition, diabetes, trauma, organ transplantation, and iron overload [1]. Mucormycosis appears to grow abundantly in iron-rich media, which predispose patients to iron overload [1]. It was recognized that the older iron chelators such as deferoxamine, when used in dialysis patients, have a higher risk for the development of zygomycosis [9,10]. Deferoxamine act as a siderophore to supply iron to the fungus, while the newer ones such as Deferasirox and Deferiprone do not facilitate iron uptake by the fungi, apparently because they share higher-affinity constants for iron, so they deprive the fungi of iron, hence its growth [9,10].
There have been few cases reported in the literature of colonic mucormycosis presenting as a colon mass. Kumar et al. reported the case of a 42-year-old diabetic patient with a right colon polypoidal mass in the ascending colon compromising the lumen, with biopsy showing a non-specific inflammatory lesion [1]. Carcinoma of the colon was one of the differentials as he underwent right hemicolectomy. Amphotericin B was not started until they received the histopathology result reporting it as mucormycosis as the patient presentation was suspicious of malignancy [1]. Chawla et al. also reported a case of Ileocolic mucormycosis mass causing intestinal obstruction; similarly, the diagnosis was made after surgical intervention, with the histopathological finding consisting of mucormycosis, resulting in delay in starting the antifungal medication [11]. Echo et al. reported a case of cecal mass 13 weeks after renal transplant. Surgical exploration reveled a perforated cecal mass, with the frozen section of the mass showing scar tissue, and could not distinguish between malignancy and fungal infection. The final pathology was consistent with mucormycosis [12]. Agha et al. reported a case of a polypoid mass in the cecum, diagnosed in colonoscopy; fortunately, the biopsy revealed it to be mucormycosis before undergoing surgical resection [8]. We noticed that colonoscopic biopsy does not always show the histology feature of mucormycosis, such as in the case reported by Kumar et al. [1]. Colonic mucormycosis presenting as a mass is extremely difficult to differentiate from carcinoma of the colon, especially when the presentation is emergent such as in Chawla et al., Echo et al., and in our case, without time for complete preoperative workup, resulting in delayed diagnosis.
In patients who received a renal transplant, malignancy is the second leading cause of death after cardiovascular disease [13]. Renal transplantation is associated with a marked increase in cancer risk at multiple sites [14]. Vajdic et al. reported elevated risk of colon cancer in dialysis patients after renal transplantation, with a relative risk of 2.36 (95% CI 1.87, 2.92) [14]. A recent meta-analysis by Wang et al. also showed elevated colon cancer risk in recipients of renal transplants (SIR: 1.85; 95% CI: 1.53–2.23; P<0.001) [15]. In general, the mean time from renal transplant to colorectal cancer diagnosis is 3.8 to 12.3 years [14].
Mucormycosis is diagnosed based on the results of histological examination or culture. Histological diagnosis is made based on the finding of predominantly aseptate hyphae, but a few reports describe the hyphae as wide and pauciseptate with focal bulbous and non-dichotomous branching, occasionally at right angles [4]. Histological examination of most samples reveal infarction and angioinvasion [5]. Histological examination in our patient revealed broad pauciseptate hyphae with evidence of thrombosis and angioinvasion of vascular wall by mucormycosis. Culture remains the main method for the identification of fungal species, even though culture results are positive in only 52% of autopsy cases and 30% of surgical specimens [6]. The low percentage of patients diagnosed based on positive fungal culture result may be related to the low index of clinical suspicion for fungal infection and the late detection of characteristic histological findings. This was the case for our patient, who presented with a colonic mass that mimicked cancer, and fungal infection was initially not suspected. He was diagnosed late because characteristic findings were detected late in the histological specimen. In some cases, the infection may be localized, and culture of the submitted specimen may not detect the pathogen [6].
Endoscopic evaluation typically reveals a dark ulcer with clearly defined borders, as well as necrosis and thrombosis in the adjacent vessels [6]. However, colonoscopy may additionally reveal hemorrhagic and edematous mucosa with erosions [16].
In the study by Almyroudis et al., 116 patients with zygomycosis diagnosed in a tertiary center were reviewed. Of these patients, 52% were diagnosed using both histopathological examination and culture, 38.8% by biopsy alone, and 6% by culture alone [3]. Diagnosis was made before death in most (86%) of the patients and postmortem in 13.8% of the patients [3]. In another review, by Song et al., of a total of 174 patients with mucormycosis who were recipients of renal transplant; 44.3% were diagnosed using both histopathological examination and culture, 41% by histopathology alone, and 14.4% by culture alone [17].
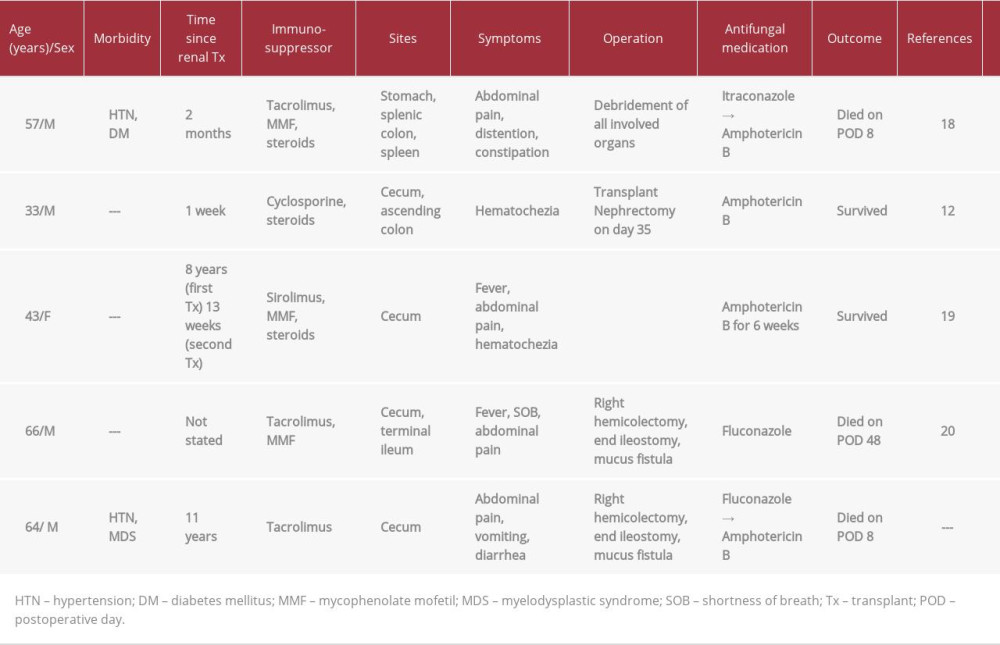
Mucormycosis is associated with high mortality of up to 50% [3]. Multiple treatment modalities have been reported in the literature, and the treatment combination of surgery and intravenous amphotericin B is associated with higher survival rates [17]. Almyroudis et al. reported that 73.7% of patients underwent surgery [3]. The mortality rate of patients who were only treated with amphotericin B was higher than that of patients who received the treatment combination of amphotericin B and surgery (62.5% versus 34.3%) [3]. Song et al. reported that 121 of the 174 patients they reviewed (69.5%) received a combination of antifungal and surgical treatment and had a higher survival rate than patients who only underwent surgery and patients who only received antifungal therapy (70.2% versus 36.4% versus 32.4%) [17]. Details of previously published reports are provided in Table 1.
In our patient, the presentation with abdominal pain with CT finding of cecal mass in association with renal transplant on immunosuppressant had a presumptive diagnosis of cecal malignancy. The patient was treated with supportive care after surgical resection. However, his condition deteriorated around the time of histopathological diagnosis of mucormycosis, 5 days after surgery. In such patients who have other risk factors, such as MDS, neutropenia, and iron overload on iron chelation therapy, it essential to have a high index of suspicion of fungal infection. If the diagnosis was suspected earlier, with the initiation of amphotericin B, the outcome of our patient might have been better.
Conclusions
In conclusion, our patient had multiple risk factors, including being a renal transplant recipient on immunosuppression, having MDS after 8 cycles of azacitidine, with history of neutropenia, and having iron overload treated with an iron chelator. We cannot precisely say which factor had the most impact, but we agree that it is multifactorial and each factor had a contribution. In a patient presenting with gastrointestinal symptoms with any of the above risk factors, the physician should be alert to the potential diagnosis of invasive mucormycosis.
We conclude that urgent surgery combined with intravenous amphotericin B is the optimal treatment option. As the symptoms of gastrointestinal mucormycosis are non-specific, diagnosis is often late or missed, and mortality remains high. High clinical suspicion, early diagnosis, and combined antifungal and surgical treatment is the best way to reduce mortality and improve survival in these patients.
Figures
References:
1.. Kumar DP, Keshari PS, Dash A, An unusual presentation of colonic mucormycosis mimicking carcinoma colon – a surgeon’s perspective: Int J Surg Case Rep, 2015; 10; 248-51
2.. Ramaswami A, Pisharam JK, Aung H, Co-incidental Plasmodium knowlesi and mucormycosis infections presenting with acute kidney injury and lower gastrointestinal bleeding: Am J Case Rep, 2013; 14; 103-5
3.. Almyroudis NG, Sutton DA, Linden P, Zygomycosis in solid organ transplant recipients in a tertiary transplant center and review of the literature: Am J Transplant, 2006; 6; 2365-74
4.. Ribes JA, Vanover-Sams CL, Baker DJ, Zygomycetes in human disease: Clin Microbiol Rev, 2000; 13; 236-301
5.. Frater JL, Hall GS, Procop GW, Histologic features of zygomycosis: Emphasis on perineural invasion and fungal morphology: Arch Pathol Lab Med, 2001; 125; 375-78
6.. Lo OS, Law WL, Ileocolonic mucormycosis in adult immunocompromised patients: A surgeon’s perspective: World J Gastroenterol, 2010; 16; 1165-70
7.. Serris A, Danion F, Lanternier F, Disease entities in mucormycosis: J Fungi, 2019; 5; 23
8.. Agha FP, Lee HH, Boland CR, Bradley SF, Mucormycoma of the colon: Early diagnosis and successful management: Am J Roentgenol, 1985; 145; 739-41
9.. Ting JY, Chan SY, Lung DC: J Pediatr Hematol Oncol, 2010; 32; 238-40
10.. Symeonidis AS, The role of iron and iron chelators in zygomycosis: Clin Microbiol Infect, 2009; 5; 26-32
11.. Chawla N, Reddy SJ, Agrawal M, Ileocolic mucormycosis causing intestinal obstruction: Indian J Med Microbiol, 2012; 30; 373-74
12.. Echo A, Hovsepian RV, Shen GK, Localized cecal zygomycosis following renal transplantation: Transpl Infect Dis, 2005; 7; 68-70
13.. Kan M, Gill JS, Wiseman SM, Colon and rectal cancer after renal transplantation: Expert Rev Anticancer Ther, 2008; 8(8); 1339-46
14.. Vajdic CM, McDonald SP, McCredie MR, Cancer incidence before and after kidney transplantation: JAMA, 2006; 296(23); 2823-31
15.. Wang Y, Lan GB, Peng FH, Xie XB, Cancer risks in recipients of renal transplants: A meta-analysis of cohort studies: Oncotarget, 2017; 9(20); 15375-85
16.. Sakorafas GH, Tsolakides G, Grigoriades K, Colonic mucormycosis: An exceptionally rare cause of massive lower gastrointestinal bleeding: Dig Liver Dis, 2006; 38; 616-17
17.. Song Y, Qiao J, Giovanni G, Mucormycosis in renal transplant recipients: Review of 174 reported cases: BMC Infect Dis, 2017; 17; 283
18.. Tinmouth J, Baker J, Gardiner G, Gastrointestinal mucormycosis in a renal transplant patient: Can J Gastroenterol, 2001; 15; 269-71
19.. Barnajian M, Gioia W, Iordache F, Bergamaschi R, Mucormycosis-induced colon perforation after renal transplantation: Surg Infect, 2014; 15; 665-66
20.. Ju JH, Park HS, Shin MJ, Successful treatment of massive lower gastrointestinal bleeding caused by mixed infection of cytomegalovirus and mucormycosis in a renal transplant recipient: Am J Nephrol, 2001; 21; 232-36
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250