16 November 2020: Articles 
Avatrombopag Optimizes Response to Niraparib by Managing Thrombocytopenia Associated with Poly-ADP Ribose Polymerase (PARP) Inhibition in Ovarian Cancer and Breast Cancer: A Case Series
Unusual or unexpected effect of treatment
Nash Gabrail1ABCDEF*, Carrie Smith1ABDOI: 10.12659/AJCR.927008
Am J Case Rep 2020; 21:e927008
Abstract
BACKGROUND: Thrombocytopenia is a potentially treatment-limiting adverse event of particular interest with the PARP inhibitor niraparib. This adverse event may necessitate niraparib dose reduction or treatment discontinuation, resulting in suboptimal treatment outcomes. Here, we report on niraparib dose optimization in 2 patients with breast cancer and 4 patients with ovarian cancer through concurrent administration of the thrombopoietin receptor stimulating agent avatrombopag to mitigate thrombocytopenia, enabling niraparib reescalation and improved clinical response.
CASE REPORT: Three of 6 patients received niraparib 300 mg daily, the highest recommended dose, for a sustained period. Avatrombopag therapy enabled niraparib dose escalation that led to reductions in biomarkers associated with disease progression. Before initiation of avatrombopag, increases in CA-125 levels, a marker for ovarian cancer, were observed in association with niraparib dose interruption, and in 2 patients with ovarian cancer CA-125 levels fell in response to niraparib dose escalation enabled by concurrent avatrombopag therapy. Further, in 2 patients with metastatic breast cancer, intracranial response was observed in association with avatrombopag-enabled niraparib therapy. In 1 patient with metastatic breast cancer, niraparib induced an intracranial response, while previous use of talazoparib had not, confirming preclinical findings of superior blood-brain-barrier penetrance with niraparib.
CONCLUSIONS: Avatrombopag is currently approved for use in chronic immune thrombocytopenia and thrombocytopenia associated with chronic liver disease in patients undergoing a surgical procedure. A clinical trial of avatrombopag for chemotherapy-induced thrombocytopenia is ongoing. Preliminary results in these 6 patient cases demonstrate the need for a confirmatory trial of avatrombopag for optimizing the dose of niraparib.
Keywords: Breast Neoplasms, Ovarian Neoplasms, Poly(ADP-ribose) Polymerases, Receptors, Thrombopoietin, Thrombocytopenia, Adenosine Diphosphate Ribose, Indazoles, Piperidines, Poly(ADP-ribose) Polymerase Inhibitors, Thiazoles, Thiophenes
Background
By interfering with DNA repair mechanisms, such as the homologous recombination repair pathway [1,2], poly-ADP ribose polymerase (PARP) inhibitors have been shown to be effective in the maintenance treatment of advanced ovarian cancer [3–8] and in advanced breast cancer with germline
Although niraparib has unique characteristics, its therapeutic efficacy may be limited by adverse events such as thrombocytopenia [14]. The FDA-approved labeling for niraparib advises interruption of niraparib therapy or dose reduction for management of thrombocytopenia, which may compromise efficacy [12,14]. Unfortunately, there are no medications currently approved for management of this potential treatment-limiting adverse event [14]. Although one agent, oprelvekin, was approved for the management of chemotherapy-induced thrombocytopenia, this agent has been discontinued [15]. Based on the mechanism of action of thrombopoietin (TPO) receptor agonists, these agents have the potential to treat thrombocytopenia associated with therapeutic agents for cancer, such as niraparib [16–18].
TPO receptor agonists increase platelet levels by interacting with the TPO receptor on megakaryocytes, the endogenous target of thrombopoietin. Given that TPO receptor agonists increase platelet production, use of these agents may counteract or mitigate PARP-induced thrombocytopenia [16]. Approved TPO receptor agonists are avatrombopag, eltrombopag, and lusutrombopag, all of which are administered orally, as well as romiplostim, which is administered as a subcutaneous injection [17–20]. Unlike eltrombopag, avatrombopag does not require regular monitoring for liver test abnormalities during therapy, which can reduce the complexity of monitoring in highly comorbid patients with cancer receiving maintenance treatment with niraparib. In addition, use of avatrombopag offers flexibility in oral dosing. Unlike eltrombopag, avatrombopag can be taken with polyvalent cations such as calcium, magnesium, and iron [17,18]. As a result, based on its mechanism of action, safety, and flexible dosing, avatrombopag is a rational choice for the prevention and management of thrombocytopenia associated with niraparib therapy.
This case series reviewed the treatment of 2 patients with breast cancer and 4 patients with ovarian cancer. All patients received niraparib for the maintenance treatment of advanced breast cancer or advanced ovarian cancer in combination with avatrombopag to reduce the risk of thrombocytopenia and improve the dose-intensity of niraparib therapy. By optimizing the dose-intensity of therapy, avatrombopag has the potential to optimize treatment outcomes, consistent with the dose-response relationship observed in multiple clinical trials of niraparib [3,6,10]. In this case series, outcomes were evaluated prospectively over the course of treatment for breast or ovarian cancer in 6 patients.
Case Reports
Case 1: Breast cancer with brain metastases
CASE 1: BREAST CANCER WITH BRAIN METASTASES:
A patient with breast cancer, born in 1977, was diagnosed with ER-positive HER2-negative stage III breast cancer in August 2016. From September 2016 to January 2017, she received 4 cycles of doxorubicin and cyclophosphamide followed by maintenance letrozole from a different provider. By January 2019, she had progressive disease with brain metastases. She received radiosurgery (Gamma Knife®) and subsequently started abemaciclib, which she did not tolerate well. Before presenting in July 2019 for her fourth opinion, she had previously been seen at multiple other centers.
From July 2019 to October 2019, she received talazoparib and experienced progressive disease in the brain with no response to therapy. Radiosurgery (Gamma Knife®) was not feasible and she declined whole-brain radiotherapy. The patient was then switched to a different PARP inhibitor, niraparib. Niraparib was chosen based on its ability to cross the blood-brain barrier and favorable results in regression of intracranial tumors in animal models [13]. The patient started treatment with niraparib 300 mg daily in November 2019, with CT-verified response in early January and late February 2020. This response was accompanied by clinical improvement, including successful discontinuation of steroids and absence of reported seizures between December 2019 and March 2020. As of her latest CT scan performed April 16, 2020, this patient has experienced complete intracranial response (Figure 1).
USE OF AVATROMBOPAG IN THIS PATIENT: Before starting therapy with avatrombopag, this patient experienced a dramatic drop in platelet count to 31 000/µL on January 8, 2020, shortly after initiating niraparib 300 mg daily on December 29, 2019. After starting treatment with avatrombopag 20 mg daily, the patient experienced a dramatic increase in platelet counts, which peaked at a supranormal level of 757 000/µL on January 23, 2020. As this patient was extremely responsive to avatrombopag, avatrombopag was held and reinitiated at a lower total weekly dose of 60 mg (20 mg administered on Monday, Wednesday, and Friday each week) starting on February 5, 2020. The patient was able to take niraparib at the maximal dose of 300 mg daily while receiving avatrombopag. Before initiating avatrombopag, niraparib 300 mg daily had previously caused profound thrombocytopenia. After initiating avatrombopag, platelet counts exceeded 250 000/µL on 3 of 5 occasions from February 5, 2020 to March 11, 2020. During this time, the patient received the maximal dose of niraparib (300 mg daily) in combination with avatrombopag 60 mg weekly (Figure 2).
CASE 2: BREAST CANCER WITH BRAIN METASTASES:
A female patient born in 1976 was diagnosed with locally advanced estrogen receptor (ER)-positive HER2-negative breast cancer in February 2016. She initially received alternative medicine. In November 2017, she sought conventional medical treatment. She had stage IV disease with liver metastases and pleural effusion and was enrolled in a clinical trial of carboplatin in combination with paclitaxel and a checkpoint inhibitor. On the clinical trial, the patient achieved partial response, until she developed progressive disease in July 2018. She received treatment with fulvestrant, palbociclib, leuprolide, and exemestane from July 2018 until the onset of progressive disease with brain metastases in April 2019. She refused cranial radiotherapy and experienced no response with single-agent doxorubicin. On May 30, 2019, she was started on niraparib and achieved partial response in the brain, liver, and lungs.
USE OF AVATROMBOPAG IN THIS PATIENT: From May 30, 2019 to March 16, 2020, the patient received weekly dose of niraparib ranging from 200 mg to 2100 mg, co-administered with avatrombopag weekly doses ranging from 140 mg weekly to 280 mg weekly. Of note, following an interruption in niraparib dosing the week of June 15, 2019, there were a total of 3 weeks in which avatrombopag was not administered (the weeks of July 29, 2019, August 26, 2019, and November 25, 2019). The gap in use of avatrombopag during July and August led to subsequent low platelet levels on of 140 000/µL on September 26, 2019 and 148 000/µL on October 21, 2019. These low platelet levels due to a gap in avatrombopag therapy led to 2 additional interruptions in niraparib therapy the weeks of November 25, 2019, and December 26, 2019. Despite the interruption of avatrombopag therapy, use of avatrombopag in this patient aided in escalating the dose of niraparib from 1800 mg weekly to 2000 mg weekly from August 29, 2019 to October 27, 2019. Most recently, the dose of avatrombopag was increased from 140 mg weekly to 280 mg weekly, resulting in a rebound in platelet counts from 48 000/µL on February 25, 2020 to 94 000/µL on March 2, 2020. The increase in avatrombopag dose also enabled escalation of the weekly dose of niraparib from 200 mg weekly to 1400 mg weekly as of March 16, 2020. On August 26, 2019 and on January 8, 2020, CT assessments indicated intracranial partial response (Figure 3). In addition to 2 standard-dose platelet transfusions, continuous administration of avatrombopag for all but 3 weeks during the dose titration helped enable administration of the optimal dose of niraparib in this patient.
CASE 3: OVARIAN CANCER:
A patient with stage IV ovarian cancer, born in 1959, was diagnosed in October 2018. She received carboplatin, paclitaxel, and bevacizumab from November 2018 to February 2019. After experiencing partial response to primary treatment, she received maintenance niraparib therapy. On her last CT scan, January 26, 2020, she was found to have stable disease. Her CA-125 levels indicate response to treatment.
USE OF AVATROMBOPAG IN THIS PATIENT: Before receiving avatrombopag, this patient was initiated on a maximal dose of niraparib of 2100 mg weekly starting on March 18, 2019, which rapidly led to a profound reduction in platelet counts, as low as 36 000/µL. Following 2 interruptions of niraparib therapy the weeks of March 25, 2019 and April 29, 2019, CA-125 levels increased from 33 units/mL to 57 units/mL. When platelet counts had recovered by May 8, 2019, the patient was again initiated on niraparib at a reduced weekly dose of 1400 mg, which gradually increased to 1900 mg weekly by October 15, 2019. Although platelet counts were initially stable during dose escalation, shortly after the dose of niraparib was escalated to 2000 mg weekly on October 19, 2019, the patient’s platelet count dropped below 100 000/µL shortly afterward on October 29, 2019. As a result, the patient was prescribed avatrombopag at a dose of 20 mg daily (140 mg weekly) to stabilize platelet counts. Initiation of avatrombopag enabled further niraparib dose escalation to 2100 mg weekly on December 15, 2019. Notably, the patient’s last platelet count was 219 000/µL on March 17, 2020 on the same dose of niraparib that had previously caused a precipitous decline in platelet counts prior to receiving avatrombopag. Additionally, CA-125 levels in this patient have more than halved since initiating treatment with avatrombopag, falling from 69 units/mL to 32 units/mL. This reduction in CA-125 levels occurred concurrently with dose escalation of niraparib to 2000 mg/week and then 2100 mg/week, which was enabled by concurrent administration of avatrombopag (Figure 4).
CASE 4: OVARIAN CANCER:
A female patient born in 1954 was diagnosed with stage II ovarian cancer concurrent with uterine cancer and fallopian tube cancer in July 2017. She received debulking surgery followed by 6 cycles of carboplatin and docetaxel from August 2017 to December 2017. In August 2019, she developed recurrence in the liver and peritoneum. Following recurrence, from August 2019 to December 2019, she received carboplatin, paclitaxel, and bevacizumab, followed by maintenance therapy with bevacizumab plus niraparib. A recent CT scan indicated stable disease.
USE OF AVATROMBOPAG IN THIS PATIENT:
In this patient, administration of avatrombopag 20 mg daily concurrently with initiation of niraparib 300 mg daily may have reduced the severity of platelet count reduction, to 63 000/µL by February 20, 2020. The use of avatrombopag 40 mg daily has enabled the patient to tolerate niraparib 300 mg daily, with a platelet count of 155 000/µL measured on March 19, 2020, 1 week after starting niraparib. CA-125 levels were stable in this patient while using niraparib in combination with avatrombopag.
CASE 5: OVARIAN CANCER:
A female patient born in 1966 was diagnosed with stage III ovarian cancer in October 2018. She received 6 cycles of carboplatin, paclitaxel, and bevacizumab from November 2018 to April 2019 followed by niraparib maintenance therapy. She declined dual maintenance therapy with bevacizumab and niraparib. Her CA-125 levels showed a rise when niraparib maintenance was interrupted, and a subsequent decline when niraparib therapy was reintroduced and the dose was escalated.
USE OF AVATROMBOPAG IN THIS PATIENT:
Before starting avatrombopag, the patient experienced 3 episodes of platelet count declines in association with niraparib use from April 2019 to August 2019 (platelet counts were 1000/µL on May 20 and May 28, 2019, 10 000/µL on July 31, 2019, and 65 000/µL on August 28, 2019). After starting avatrombopag at a weekly dose of 140 mg, the patient was able to tolerate an increase in her weekly niraparib dose from 500 mg to 900 mg on December 2, 2019 and a further increase to 1000 mg weekly on March 18, 2020. On February 5, 2019, the dose of avatrombopag increased from 140 mg weekly to 280 mg weekly. Despite continued up-titration in the dose of niraparib, platelet counts continued to rise. CA-125 levels fell from 43 U/mL on February 5, 2020 to 37 U/mL by March 4, 2020 concurrent with continuous use of niraparib and avatrombopag in this patient.
CASE 6: OVARIAN CANCER:
A patient born in 1944 was diagnosed with stage III ovarian cancer in March 2019. From March 2019 to May 2019, she received 4 cycles of carboplatin, paclitaxel, and bevacizumab, followed by debulking surgery in June 2019. Following disease progression, she received 4 cycles of carboplatin and paclitaxel until September 2019, followed by rucaparib maintenance. CA-125 levels in this patient had normalized since surgical intervention and have remained stable throughout treatment. As of March 2020, she was disease-free.
USE OF AVATROMBOPAG IN THIS PATIENT:
In this patient, niraparib therapy was initiated at a dose of 700 mg weekly on December 4, 2019, which was gradually increased to 2100 mg weekly by January 7, 2020. With upward dose titration, platelet counts fell from a peak of 199 000/µL on December 11, 2019 to 106 000/µL by December 26, 2019. As a result of this drop in platelet counts, avatrombopag was also initiated on December 26, 2019 at a daily dose of 20 mg (140 mg weekly). As a result of avatrombopag coadministration, the patient experienced an increase in platelet counts to 163 000/µL on January 7, 2020. To support further dose escalation and platelet count stabilization on January 7, 2020, the daily dose of avatrombopag was escalated to 40 mg daily (280 mg weekly) and the niraparib dose was escalated to the maximal dose of 2100 mg weekly. Although 2 troughs in platelet levels occurred despite avatrombopag therapy (47 000/µL on January 29, 2020 and 28 000/µL on March 4, 2020), these troughs were managed through niraparib dose reduction initially to 1400 mg weekly on January 29, 2020, and then to 700 mg weekly by March 11, 2020. Although dose reductions were necessary to maintain platelet counts above 100 000/µL, this patient has been able to continue niraparib therapy, which may not have been possible in the absence of avatrombopag therapy.
Results
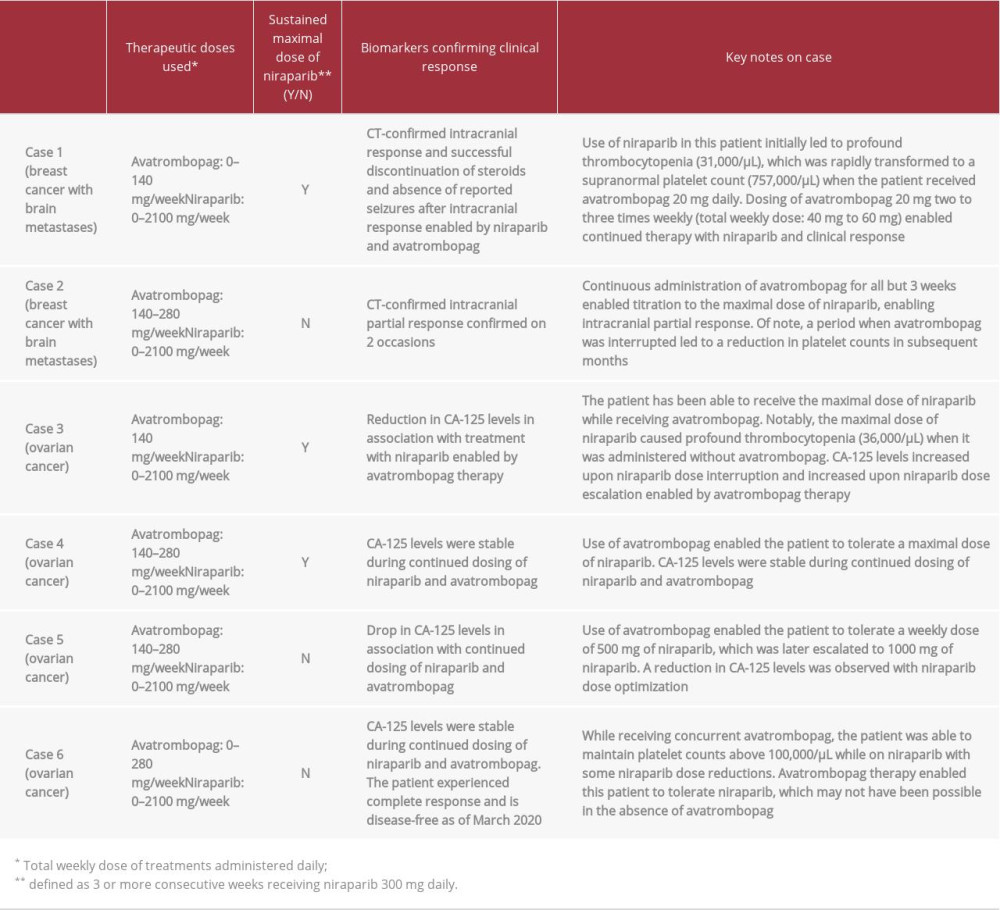
Three of 6 patients were able to sustain treatment with the maximum dose of niraparib (300 mg daily) for at least 3 continuous weeks. Continuous administration of avatrombopag mitigated thrombocytopenia and enabled dose escalation of niraparib to induce CT-confirmed response in 2 patients with breast cancer (cases 1 and 2). Reductions in CA-125 levels were observed in association with niraparib therapy enabled by avatrombopag therapy in 2 of 4 patients with ovarian cancer (cases 3 and 5). The remaining 2 patients had stable CA-125 levels (cases 4 and 6). One of these patients (case 6) has experienced complete response and was disease-free as of March 2020. One patient with breast cancer who experienced profound thrombocytopenia (31 000/µL) on niraparib attained supranormal platelet counts (757 000/µL) within 3 weeks of initiating avatrombopag 20 mg daily and was transitioned to a reduced dose (20 mg 3 times weekly), which enabled continued therapy with niraparib and continued response (Table 1).
Discussion
Use of niraparib is limited by potential adverse events, including thrombocytopenia [14], which can lead to dose interruption or treatment discontinuation, which may reduce efficacy [12,14]. With a lack of approved medications for management of thrombocytopenia, there is a need for new therapeutic options [14,15]. As demonstrated in this case series, the TPO receptor agonist avatrombopag can counteract thrombocytopenia by interacting with the TPO receptor on megakaryocytes, the endogenous target of thrombopoietin [16–18]. In this case series of 2 patients with breast cancer and 4 patients with ovarian cancer, concurrent administration of the TPO receptor agonist avatrombopag mitigated thrombocytopenia and enabled both niraparib dose reescalation and improved clinical response.
In 2 patients with metastatic breast cancer, intracranial response was observed in association with avatrombopag-enabled niraparib therapy. Notably, in 1 patient with metastatic breast cancer, niraparib induced intracranial response, while previous use of talazoparib had not. This observation is confirmatory of preclinical findings with niraparib indicating superior blood-brain-barrier penetrance over other PARP inhibitors [13].
To reduce the risk of adverse events with niraparib, including thrombocytopenia, the starting dose of niraparib may be adjusted based on baseline bodyweight and platelet count. Specifically, patients with a baseline bodyweight <77 kg or with a baseline platelet count <150 00/µL may receive a reduced starting dose of 200 mg of niraparib daily, rather than a starting dose of 300 mg daily. In the PRIMA trial evaluating niraparib in the first-line maintenance setting of ovarian cancer, this strategy was introduced as a protocol amendment, and was shown to reduce the risk of thrombocytopenia from 52.4% with fixed starting dose niraparib to 33.7% with individualized starting dose niraparib. However, the initial dose adjustment of niraparib does not eliminate the issue of thrombocytopenia, as 14.8% of patients receiving individualized starting dose niraparib in PRIMA experienced thrombocytopenia of grade 3 severity or higher. Based on this case series, the risk of developing treatment-limiting thrombocytopenia may be further reduced through the use of avatrombopag in appropriate patients [19,20].
In patients with ovarian cancer, prior to avatrombopag initiation, increases in CA-125 levels in association with niraparib dose interruption occurred as a result of suboptimal niraparib dosing in association with thrombocytopenia. Additionally, in 2 patients with ovarian cancer, CA-125 levels fell in response to niraparib dose escalation enabled by concurrent avatrombopag therapy. This result indicates a potential for improved clinical outcomes in association with niraparib dose optimization enabled by avatrombopag therapy. Preliminary results in these 6 patient cases demonstrate the need for a confirmatory trial of avatrombopag for optimizing the dose of niraparib.
Conclusions
Responses in this small series of patients indicate a role for avatrombopag in optimizing the dose of niraparib in patients receiving maintenance treatment for ovarian cancer and breast cancer. In this case series, the adverse event profile of niraparib did not differ from those noted for avatrombopag in trials supporting its indication for use in immune thrombocytopenia. One potential limitation of this case series is the fact that thrombocytopenia induced by PARP inhibition might not share the same bone marrow suppression effect observed with chemotherapy-induced thrombocytopenia. Avatrombopag should be further investigated in thrombocytopenia induced by niraparib in a trial assessing bone marrow for megakaryocyte count over the course of niraparib therapy. These preliminary results, presented in the form of a case series, demonstrate the need for a large prospective trial to confirm our findings.
Figures
References:
1.. Sonnenblick A, de Azambuja E, Azim HA, Piccart M, An update on PARP inhibitors – moving to the adjuvant setting: Nat Rev Clin Oncol, 2015; 12(1); 27-41
2.. Weil MK, Chen AP, PARP inhibitor treatment in ovarian and breast cancer: Curr Probl Cancer, 2011; 35(1); 7-50
3.. Mirza MR, Monk BJ, Herrstedt J, Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer: N Engl J Med, 2016; 375(22); 2154-64
4.. González-Martín A, Pothuri B, Vergote I, Niraparib in patients with newly diagnosed advanced ovarian cancer: N Engl J Med, 2019; 381(25); 2391-402
5.. Moore K, Colombo N, Scambia G, Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer: N Engl J Med, 2018; 379(26); 2495-505
6.. Moore KN, Secord AA, Geller MA, Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial [published correction appears in Lancet Oncol, 2019; 20(5): e242]: Lancet Oncol, 2019; 20(5); 636-48
7.. Pujade-Lauraine E, Ledermann JA, Selle F, Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial [published correction appears in Lancet Oncol, 2017; 18(9): e510]: Lancet Oncol, 2017; 18(9); 1274-84
8.. Coleman RL, Oza AM, Lorusso D, Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial [published correction appears in Lancet, 2017; 390(10106): 1948]: Lancet, 2017; 390(10106); 1949-61
9.. Litton JK, Rugo HS, Ettl J, Talazoparib in patients with advanced breast cancer and a germline BRCA mutation: N Engl J Med, 2018; 379(8); 753, doi: 10.1056/NEJMoa1802905 –. doi:
10.. González-Martín A, Pothuri B, Vergote I, Niraparib in patients with newly diagnosed advanced ovarian cancer: N Engl J Med, 2019; 381(25); 2391-402
11.. , FDA approves niraparib for first-line maintenance of advanced ovarian cancer, 2020 https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer
12.. Wang J, Zhang Z, Mirza MR, The exposure-response relationship of niraparib in patients with gBRCAmut and non-gBRCAmut: Results from the ENGOT-OV16/NOVA trial: ESMO 2017 Congress Sep 8–12, 2017, Madrid, Spain https://oncologypro.esmo.org/meeting-resources/esmo-2017-congress/The-Exposure-Response-Relationship-of-Niraparib-in-Patients-with-gBRCAmut-and-Non-gBRCAmut-Results-from-the-ENGOT-OV16-NOVA-Trial
13.. Sun K, Mikule K, Wang Z, A comparative pharmacokinetic study of PARP inhibitors demonstrates favorable properties for niraparib efficacy in preclinical tumor models: Oncotarget, 2018; 9(98); 37080-96
14.. : ZEJULA (niraparib) [package insert], 2020, Research Triangle Park, NC, GlaxoSmithKline
15.. , FDA Drug Shortages https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Oprelvekin%20(Neumega%C2%AE)%20Lyophilized%20Powder%20for%20Injection%20Rx&st=d&tab=tabs-4
16.. Zufferey A, Kapur R, Semple JW, Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP): J Clin Med, 2017; 6(2); 16
17.. : DOPTELET (avatrombopag) [package insert], 2019, Durham, NC, Dova Pharmaceuticals Inc
18.. : PROMACTA (eltrombopag) [package insert], 2018, East Hanover, NJ, Novartis Pharmaceuticals Corporation https://www.accessdata.fda.gov/drug-satfda_docs/label/2018/022291s021lbl.pdf
19.. Berek JS, Matulonis UA, Peen U, Safety and dose modification for patients receiving niraparib: Ann Oncol, 2018; 29(8); 1784-92
20.. González-Martín A, Pothuri B, Vergote I, Niraparib in patients with newly diagnosed advanced ovarian cancer: N Engl J Med, 2019; 381(25); 2391-402
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250