25 September 2020: Articles 
Nosocomial COVID-19 Infection and Severe COVID-19 Pneumonia in Patients Hospitalized for Alcoholic Liver Disease: A Case Report
Rare coexistence of disease or pathology
Hanna Wiśniewska1ABDEF, Miłosz Skowron2ABDEF, Dorota Bander1AB, Monika Hornung1AB, Krzysztof Jurczyk1AB, Ewa Karpińska1AB, Łukasz Laurans1AB, Łukasz Socha1AB, Zenon Czajkowski2AB, Marta Wawrzynowicz - Syczewska1ABEFG*DOI: 10.12659/AJCR.927452
Am J Case Rep 2020; 21:e927452
Abstract
BACKGROUND: COVID-19 is an infectious disease caused by SARS-CoV-2. It has spread rapidly through the world, endangering human life. The main target of COVID-19 is the lungs; however, it can involve other organs, including the liver. Patients with severe COVID-19 have an increased incidence of abnormal liver function, and patients with liver disorders are considered to be at a higher risk of severe COVID-19 infection. The mechanism of liver injury reported in 14% to 53% of COVID-19 patients is poorly recognized and several possibilities need to be considered (cytokine storm, direct viral action, hypoxia). The incidence of underlying liver comorbidities in patients with a COVID-19 infection ranges from 1% to 11%.
CASE REPORT: This is a report of 2 nosocomial COVID-19 infections and severe COVID-19 pneumonia in 2 patients who were hospitalized during treatment for alcoholic liver disease (ALD). Case 1 and case 2 were a 31-year-old woman and a 40-year-old woman, respectively, with decompensated ALD and symptoms of the COVID-19 infection. Both patients were transferred from another hospital to our hospital after confirmation of COVID-19 during their hospitalization. The course of the infection progressed rapidly in both patients with the development of multiple-organ failure and death over a short period.
CONCLUSIONS: There are no clear recommendations on the management of ALD in the COVID-19 pandemic. Alcoholic hepatitis may be a risk factor for severe COVID-19 and a poor outcome. A high percentage of nosocomial COVID-19 infections are observed; therefore, special precautions should be taken to minimize the risk of COVID-19 exposure.
Keywords: SARS Virus, COVID-19, Hepatitis, Alcoholic, Liver Diseases, Alcoholic, Cross Infection, case reports, COVID-19, Combined Modality Therapy, Coronavirus Infections, Disease Progression, Fatal Outcome, Hospitalization, Multiple Organ Failure, Pandemics, Pneumonia, Viral, Radiography, Thoracic, Respiration, Artificial, Risk Assessment, severe acute respiratory syndrome, Tomography, X-Ray Computed
Background
COVID-19 is an infectious disease caused by β-coronavirus, a positive-sense single-strand RNA (+ssRNA) virus, officially named SARS-CoV-2 (severe acute respiratory syndrome corona-virus 2) [1–5]. COVID-19 has spread rapidly through the world, endangering human life [1–5]. On March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic [3,5]. On August 16, 2020, the total number of COVID-19 cases was 21 294 845 with 761 779 deaths (mortality rate 3.58%). In Poland, there were 56 090 confirmed cases with 1869 deaths (mortality rate 3.33%). The true mortality rate of COVID-19 is still unknown [6]. There are 2 methods of COVID-19 transmission: person-to-person (direct contact, most often via small droplets produced by coughing, sneezing, talking) or indirect transmission (non-contact, by contaminated surfaces and objects). The incubation period ranges from 1 to 14 days, and is usually between 3 to 7 days [3]. SARS-CoV-2 is similar to SARS-CoV (severe acute respiratory syndrome coronavirus) and MERS-CoV (Middle East respiratory syndrome-related coronavirus). SARS-CoV-2, when compared to SARS-CoV and MERS-CoV, is less virulent but more infectious. It has greater epidemic potential because it is difficult to trace mild or presymptomatic infections [2,3,5].
COVID-19 uses the angiotensin-converting enzyme 2 (ACE2) as a cellular receptor, which is a protein distributed on the surface of many types of cells in the human body, including the liver [7–9]. The lungs are the main target of COVID-19; however, it can also involve other organs [3,10]. The liver is a potential or direct target of infection [9]. Hepatocytes and cholangiocytes express the ACE2 receptor necessary for cell entry [9,11]. The COVID-19 virus probably replicates within the hepatocytes. Abnormal liver function or liver damage, either in the form of hepatitis and/or cholestasis, can be observed in patients with COVID-19 [8]. After infecting the target cells, the COVID-19 virus takes control of their DNA, replicates itself, and spreads to other cells. The symptoms of COVID-19 range from asymptomatic cases to mild (as a self-limiting respiratory disease) to extremely severe progressive pneumonia, which can be fatal [3–5]. Most people infected by COVID-19 have mild symptoms [4,5]. The major clinical feature of the COVID-19 infection is virus-associated pneumonitis. The typical clinical manifestations of COVID-19 are fever, dry cough, fatigue, shortness of breath, no improvement on antibiotic treatment, the loss of sense of taste or smell, a low white blood cell (WBC) count (neutropenia and lymphopenia), lung inflammation, and elevated levels of aminotransferases [3,5,8]. The severity of liver dysfunction increases with the severity of COVID-19 [7]. Patients with acute liver disease or decompensated cirrhosis could be at an increased risk of a COVID-19 infection due to immune dysfunction, and have poor outcomes after COVID-19 infections [7,11]. Chronic liver disease is associated with higher mortality after a COVID-19 infection. The incidence of underlying liver comorbidities in patients with COVID-19 infections range from 1% to 11% [9,12,13]. The hospitals experienced many cases of nosocomial COVID-19 infections. Around 12% to 15% of COVID-19 cases were originally admitted to hospital for reasons other than COVID-19 infections and became infected during their hospital stay [14].
In this report, we present 2 cases of nosocomial COVID-19 infections and severe COVID-19 pneumonia in 2 women who were hospitalized during treatment of alcoholic liver disease (ALD).
Case Reports
CASE 1:
A 31-year-old woman with ALD was admitted to our hospital because of dyspnea, cough, fever, and suspicion of COVID-19-induced pneumonia. She also presented with symptoms of liver decompensation. Prior to admission, she was hospitalized in another local hospital with signs and symptoms suggesting alcoholic hepatitis. These symptoms were jaundice, hepatomegaly, tense ascites, peripheral edema, increased liver enzyme activity with the predominance of aspartate aminotransferase (AST) over alanine aminotransferase (ALT) (AST/ALT ratio >2), high gamma glutamyl transferase (GGT) activity, macrocytic anemia, elevated serum bilirubin, increased serum triglycerides, decreased serum albumin, an elevated white blood cell count, and coagulopathy [15]. After several days of hospitalization, new symptoms, including fever, dyspnea, and cough, appeared. The chest X-ray was normal. Due to the epidemio-logical situation of that local hospital (a cluster of COVID-19 infections), the patient underwent a nasopharyngeal swab and tested positive. The COVID-19 infection was confirmed by the reverse transcription-polymerase chain reaction (RT-PCR) test from the nasopharyngeal swab.
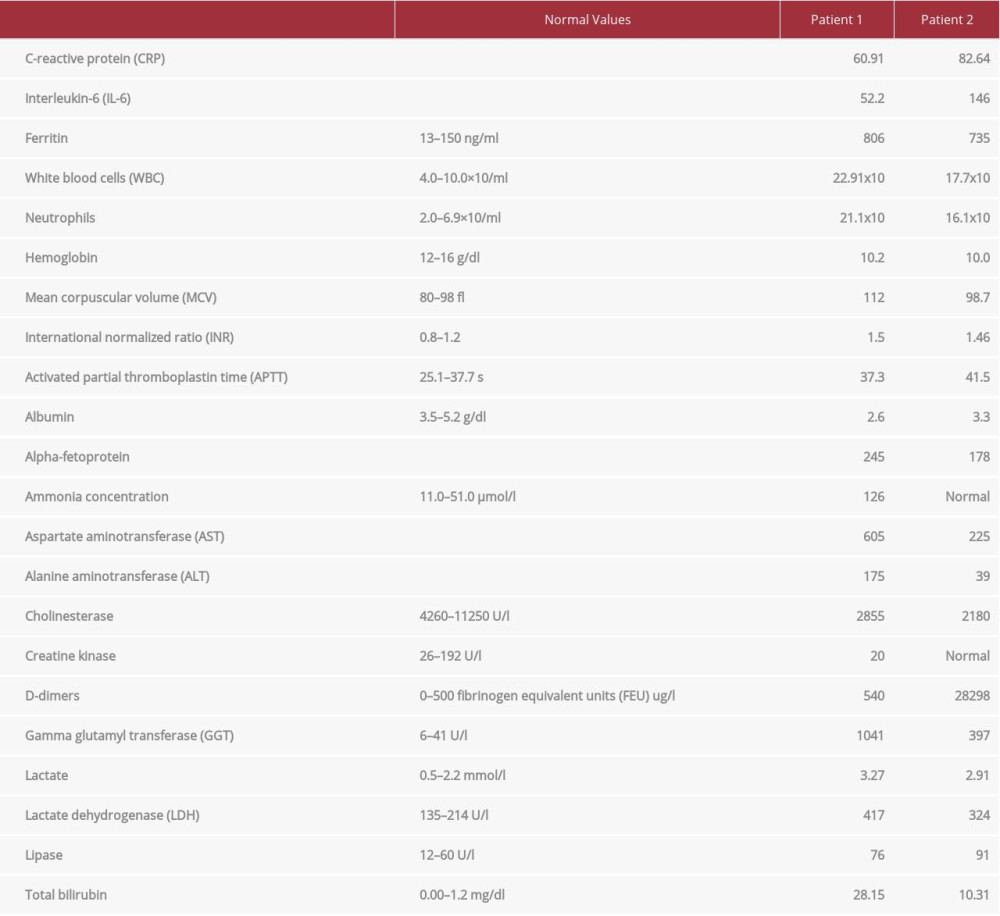
She was transferred to our hospital with a suspicion of COVID-19-associated pneumonia. Her medical history was remarkable for hypertension, 2 cesarean sections, cholecystectomy, diagnostic laparoscopy, and recent gastrointestinal bleeding probably related to excessive alcohol intake. The patient elicited a history of 4-year habitual alcohol consumption (mainly vodka and vodka drinks). There were no cases of alcohol abuse in the patient’s family. Her family’s medical history was insignificant. At admission, she was conscious and coherent, and complained of cough and dyspnea. Her vital signs were a temperature of 37.3°C, blood pressure 120/70 mmHg, pulse 95/min, and an oxygen saturation of 90%. Her physical examination was remarkable for jaundice, a tense abdominal wall due to ascites, significant hepatomegaly, and a palpable spleen. Bowel sounds were present; there was neither rebound nor guarding. Percussion and auscultation of the lungs were normal. The laboratory tests showed elevated inflammatory parameters and abnormalities caused by alcohol-induced liver damage. Table 1 shows the laboratory findings from her test results. Tests for hepatitis A, B, and C viruses (HAV, HBV, HCV) and the human immunodeficiency virus (HIV) were also performed. Acute and chronic hepatitis B and C, as well as an HIV infection, were excluded. The abdominal ultrasound showed ascitic fluid and an enlarged steatotic liver of heterogeneous and fine-grained echogenicity. The unenhanced computed tomography (CT) scan of the thorax showed bipulmonary ground-glass opacities predominant on the left side, with transition to consolidations compatible with COVID-19 viral pneumonia. There was a moderate amount of pleural effusion (ascitic hydrothorax) on the right side (Figure 1). Due to dyspnea, she received oxygen supplementation and pulse oximetry initially increased to 95% to 96%. Soon after admission, an intensive care unit (ICU) practitioner assessed the patient’s respiratory condition and her requirements for intensive care management; however, she did not initially meet the ICU admission criteria. She gave written consent for treatment with lopinavir/ritonavir and tocilizumab. Her medications were chloroquine (500 mg twice daily, oral), azithromycin (500 mg once daily, oral), ceftriaxone (2 g once daily, intravenous), albumin infusions (initially 20 g twice daily, intravenous), diuretics (spironolactone 200 mg daily, intravenous; furosemide 80 mg per day, oral), L-aspartate-L-ornithine infusions (1 g twice daily, intravenous), and hepatoprotective drugs (silibinin 140 mg 3 times a day, oral; ornithine aspartate 3 g twice daily, oral). Lopinavir/ritonavir (400 mg/100 mg twice daily, oral) was added the next day. As this patient was treated in April 2020, we had little experience in treating patients with COVID-19. Lopinavir/ritonavir is contraindicated in severe liver failure; therefore, there was concern whether the use of this drug would cause a deterioration in her liver function.
On the 3rd day of hospitalization, there was rapid deterioration. She could not tolerate a non-rebreather mask, pulse oximetry decreased to 80% to 85%, rhonchi and crackles appeared during the lung auscultation, and the abdominal swelling worsened. Due to respiratory failure, she was intubated by the ICU practitioner prior to ICU admission, and received invasive mechanical ventilation with 100% oxygen and deep sedation. Arterial blood gases (ABG) after admission to the ICU revealed pO2 (partial pressure of oxygen) to be 118.1 mmHg (PaO2: FiO2 ratio [arterial oxygen partial pressure to fractional inspired oxygen ratio]=118). This was consistent with moderate acute respiratory distress syndrome (ARDS) [16]. In the ICU, the patient was mechanically ventilated in the BIPAP (bilevel positive air pressure) mode (Drager Evita XL, Drägerwerk AG & Co. KGaA, Lubeck, Germany) with low tidal volume. During treatment, the inspiration pressure (Pins) ranged from 28 to 32 cmH2O and the positive end-expiratory pressure (PEEP) ranged from 10 to 14 cmH2O. Recruitment maneuvers were applied. The FiO2 on the first day of mechanical ventilation was 0.8–1.0 and improved on day 5 to 0.75 (PaO2: FiO2 ratio=142). She required vasopressors (norepinephrine) due to hemodynamic instability. Diuretics were administered in the continuous intravenous injection. Blood and endotracheal aspirate cultures were negative. Enteral nutrition was administered; however, on day 4 of the ICU stay, total parenteral nutrition was started due to active gastrointestinal bleeding. Despite intensive treatment, further increases of the inflammatory parameters were observed and her body temperature increased to 40°C. The patient received 600 mg of tocilizumab infusion, repeated on 2 consecutive days. On the 5th day, the antibiotic regime was modified; ceftriaxone was replaced by meropenem (1 g 3 times daily, intravenous), linezolid (600 mg twice daily, intravenous), rifaximin (400 mg 3 times daily, oral) and fluconazole (200 mg per day, intravenous). An antiarrhythmic drug (amiodarone) was introduced. On the 7th day, gastrointestinal hemorrhage was observed (hemoglobin decreased to 6.3 g/dl), and she required a transfusion of blood products (4 units of packed red blood cells [RBC] and 4 units of fresh-frozen plasma). Continuous intravenous injection of a proton pump inhibitor, tranexamic acid, and cyclonamine were used to stop the active gastrointestinal bleeding. Albumin infusions were continued. In the following days, she required another transfusion of RBC; she became hyperpyretic (41°C) with no response to the antipyretic drugs and physical cooling. Her serum level of interleukin-6 (IL-6) increased to 941 pg/ml. Severe impairment was observed in the coagulation parameters such as activated partial thromboplastin time (APTT) and international normalized ratio (INR), as well as a significant increase in the ammonia levels. Finally, she developed intractable hepatorenal syndrome, and the hemodynamic instability worsened. She died of multi-organ failure on the 9th day of hospitalization. We have obtained consent from the next of kin for publication of this case report and approval from the Bioethics Committee (consent number: KB-0012/38/2020).
CASE 2:
A 40-year-old woman was admitted to the local hospital because of jaundice, ascites, and fever. Based on the abdominal ultrasound showing liver steatosis, ascites, and hepatomegaly, as well as the laboratory abnormalities typical for alcoholic liver injury, she was diagnosed with alcoholic hepatitis with liver decompensation. The symptoms were jaundice, hepatomegaly, ascites, peripheral edema, increased aminotransferases with the predominance of AST (AST/ALT ratio >2), high GGT activity, macrocytic anemia, elevated serum bilirubin, increased serum triglycerides, decreased serum albumin, an elevated WBC count, and coagulopathy [15]. Viral infections such as HAV, HBV, HCV, and HIV were excluded after standard tests. After 14 days of hospitalization, due to the epidemiological situation at the department (a cluster of COVID-19 infections), the patient underwent a nasopharyngeal swab for a COVID-19 infection and tested positive. COVID-19 was confirmed by the RT-PCR test.
At admission to our hospital, she was conscious and coherent; she had jaundice, complained of a dry, non-productive cough for the past 2 days, and had dark urine. She reported excessive alcohol consumption, mostly wine and vodka drinks, since her youth, as well as previous hospitalizations related to alcohol abuse. Her past medical and family histories were unremarkable for chronic illnesses. There were no cases of alcohol abuse in her family. Vital signs at admission were a temperature of 37.7°C, blood pressure 140/80 mmHg, pulse 110/min, and oxygen saturation 90%. On physical examination, she presented with multiple spider nevi on the chest skin, jaundice, obesity (BMI 38.06 kg/m2), ascites, and leg swelling. Percussion and auscultation of the lungs were normal. The abdominal wall was elevated and tense due to ascites. The liver was grossly enlarged and tender, and the spleen was not palpable. Bowel sounds were present; there was neither rebound nor guarding. Laboratory tests showed elevated inflammatory parameters and abnormalities typical for ALD (Table 1) [11].
An unenhanced CT scan of the thorax revealed bipulmonary ground-glass opacities and a crazy-paving appearance predominant on the left side, compatible with COVID-19 viral pneumonia (Figure 2). A CT scan of the abdomen showed splenomegaly and an enlarged, steatotic liver surrounded by ascitic fluid. Oxygen saturation decreases below 88% to 90% were noted and she received oxygen supplementation with an initial good result (pulse oximetry increased to 94%). An ICU practitioner assessed the patient’s respiratory condition and her requirements for intensive care management; however, initially, she did not meet the ICU admission criteria. The patient gave written consent for treatment with lopinavir/ritonavir and tocilizumab. As treatment, she received chloroquine (500 mg twice daily, oral), azithromycin (500 mg once daily, oral), ceftriaxone (2 g once daily, intravenous), albumin infusions (20 g once daily, intravenous), diuretics (spironolactone 200 mg daily, intravenous; furosemide 80 mg per day, oral), vitamin K supplementation, hepatoprotective drugs (silibinin 140 mg 3 times daily, oral; ornithine aspartate 3 g twice a day, oral), and subsequently lopinavir/ritonavir (400 mg/100 mg twice daily, oral) and steroids.
On the 4th day, following admission to the COVID-19 hospital, rapid deterioration was observed. The pulse oximetry decreased below 88%, rhonchi and crackles appeared during lung auscultation, and the ascites increased. Due to respiratory failure, the patient was intubated by an ICU practitioner prior to ICU admission, and she received invasive mechanical ventilation with 100% O2 and deep sedation. The ABG after admission to the ICU revealed pO2 59.7 mmHg (PaO2: FiO2 ratio=59.7). This ratio was consistent with severe ARDS [16]. In the ICU, she was mechanically ventilated in the BIPAP mode (Drager Evita XL, Drägerwerk AG & Co. KGaA, Lubeck, Germany) with low tidal volume. During treatment, Pins ranged from 28 to 34 cmH2O and PEEP ranged from 12 to 15 cmH2O. Recruitment maneuvers were applied. The neuromuscular blockade had to be used due to severe hypoxemia. The FiO2 on day 1 of the mechanical ventilation was 0.9–1.0 and improved on day 5 to 0.75 (PaO2: FiO2 ratio=97.8). She required vasopressors (norepinephrine) as she developed hemodynamic instability. Initially, diuretics were given in fractional doses and then in the continuous intravenous injection. Blood and endotracheal aspirate cultures were negative. Enterococcus faecium HLAR(+) (high-level aminoglycoside resistance) VRE(+) (vancomycin-resistant Enterococcus) sensitive to linezolid was recovered from the rectal swab. In the ICU, enteral nutrition was administered; however, partial parenteral nutrition had to be added as she was unable to achieve adequate nutrition. She developed ascitic hydrothorax of the right pleura. An ultrasound-guided pleural cavity drainage was performed and 5.7 L of clear, straw-colored pleural fluid was aspirated. It resulted in a temporary improvement of gas exchange (FiO2 improved to 0.8). Further increases in the inflammatory parameters were noted, IL-6 peaked to 1156 pg/mL, and the leukocyte count increased to 35.5×103/µl. CRP showed a downward trend and the procalcitonin level was normal. The antibiotic regime was changed; ceftriaxone was replaced by meropenem (1g 3 times daily, intravenous) and linezolid (600 mg twice a day, intravenous). The dosage of vasopressors had to be increased. Severe anemia was observed, and she required a transfusion of blood products (2 units of packed RBC). The patient became hyperpyretic (39.9°C). Tachyarrhythmia appeared. She developed massive ascites requiring paracentesis (2 L of bloody fluid was drained). Blood appeared in the nasogastric tube, indicating gastrointestinal hemorrhage, even though APTT and INR were only slightly elevated. Due to the impaired gas exchange, on the 12th day of ICU hospitalization, a percutaneous tracheostomy was performed; however, she showed no improvement. Despite intensive therapy, she developed uncontrollable hemodynamic instability and profound hypoxia. She died of cardiopulmonary insufficiency on the 12th day of the ICU hospitalization. We have obtained consent from the next of kin for publication of this case report and approval from the Bioethics Committee (consent number: KB-0012/40/2020; KB-0012/117/2020).
Discussion
Alcohol is a major hepatotoxin and more than 40% of all deaths from liver disease can be attributed to alcohol consumption. Alcoholic hepatitis represents a wide spectrum of liver disease ranging from mild to a severe life-threatening multi-organ injury, especially in patients who have already developed liver cirrhosis. Female sex and younger age are risk factors for alcoholic hepatitis. Lower amounts of alcohol and shorter durations of abuse are as harmful for women as higher amounts of alcohol and longer durations of abuse [15,17]. The current epidemiological crisis caused by COVID-19, with prolonged social isolation, fear and anxiety, and economic instability, has resulted in excessive alcohol consumption worldwide. Patients with alcohol-related liver disorders can be particularly vulnerable to a relapse during the pandemic since in-person support groups such as Alcoholics Anonymous (AA) are not available [18]. Moreover, in-person visits and consultations for outpatients with liver disease were limited or even halted for an indeterminate period, negatively affecting the quality of care in hepatology. In turn, we can expect an increasing number of liver decompensations, including patients with severe alcoholic hepatitis who returned to addiction [12].
The diagnosis of ALD is based on medical history, clinical symptoms, and laboratory abnormalities [15,19]. The short-term prognosis in alcoholic hepatitis is estimated by using a simple formula, called the Maddrey discriminant function (DF) score. Patients with a DF score of >32 have a 50% mortality rate during their current hospitalization [20]. In both the presented cases, the DF score was elevated, indicating poor prognoses (the DF of patient 1 was 47.8, the DF of patient 2 was 33.5). The only effective treatment in decompensated patients is liver transplantation; however, it can only be offered to patients in whom abstinence from alcohol for at least 6 months has not resulted in spontaneous improvement and who declare long-term sobriety [15,21]. Without any professional support, remaining sober for so long can be difficult for many patients addicted to alcohol. In the COVID-19 era, this issue became a critical and challenging area for which protocols and policies probably need to be carefully reconsidered.
Patients with chronic liver disease represent a population at a higher risk of acquiring COVID-19 and suffering from complications [18]. Patients with pre-existing liver disease and COVID-19 were at increased risk of mortality as compared to patients without liver disease, and the relative risk was markedly higher in patients with cirrhosis [22]. There is scarce information on the interaction of alcoholic hepatitis and COVID-19 [23]; however, it is well-known that patients with advanced ALD are more likely to develop various forms of respiratory distress: pneumonia, ARDS, rhinosyncytial virus infection, and a more aggressive course of influenza [24]. Alcohol itself interferes with the immune system and compromises its function [25]. In the 2 presented cases, alcoholic hepatitis was a risk factor for the severe course of COVID-19 and the poor outcomes as both patients developed severe interstitial pneumonia with the involvement of 2 lobes, ground-glass opacities, and a crazy-paving pattern, and finally full-blown ARDS requiring intensive care treatment. So far, we have not seen such a rapid course of the disease among our patients. Both the patients described in this case report were primarily hospitalized elsewhere. That hospital was recognized as one of the COVID-19 outbreak centers in the West Pomeranian Voivodeship. It cannot be ruled out that the virus was more ‘virulent’ there.
There are no clear recommendations on the management of alcoholic liver disease in the COVID-19 pandemic [12]. There are a few aspects to be considered: respiratory failure, liver failure in the course of the underlying chronic disorder, and the COVID-19 infection itself, which can cause liver injury via 3 possible mechanisms (cytokine storm, direct viral action, and hypoxia) [7,25–27]. Liver impairment is a common observation among patients with a COVID-19 infection. Chronic liver diseases are associated with higher mortality after a COVID-19 infection. Therefore, there should be regular monitoring of liver biochemistries for all patients with COVID-19 [7,9]. We need further research to understand the impact of this infection on the body. For this reason, close observation, reporting, and special precautions should be taken when treating patients, especially those with liver disease [7]. Systematic viral infections are often associated with transient elevations of transaminases, which may reflect general immune activation or inflammation caused by circulating cytokines without compromising liver function, a phenomenon called ‘bystander hepatitis.’ Possibly, patients with advanced chronic liver disease and COVID-19 are at increased risk of infection due to immune dysfunction [8].
Our treatment was based on internationally approved guidelines with the implementation of local protocols. The low tidal volume was an essential part of the mechanical ventilation [24–26]. To improve the ventilation and gas exchange, recruitment maneuvers were provided to both patients and a neuromuscular blockade was provided to patient 2 with severe hypoxia [28–31]. The steroids for treating alcoholic hepatitis, considered in case of high DF scores, were contraindicated in both patients due to active bleeding. In a contentious issue regarding the SARS-CoV-2 treatment protocol [14,24], we decided to follow the national guidelines for oxygen-dependent symptomatic COVID-19, and lopinavir/ritonavir was added to the medication list [5]. The use of tocilizumab is still controversial and restricted to clinical trials.
Appropriate procedures should be implemented during each hospitalization, beginning with the use of surgical masks by staff and patients and frequent hand washing. Accompanying visitors should not be allowed for adult patients. All suspected or confirmed cases who meet the COVID-19 management guidelines should be hospitalized in an independent building dedicated to COVID-19 patients [9]. Patients who use the health care system are more likely to contract severe COVID-19. Research indicates that comprehensive infection prevention and control measures reduce the number of nosocomial infections [11].
Generally, current recommendations for patients with chronic liver disorders are to stay calm, stay at home, and continue their current regimen and recommendations [18]. However, there are many concerns and doubts about reopening care in hepatology. These include a timetable to restart treatment for chronic liver disease patients, identifying those who need more attention, the hospitalization of alcoholic hepatitis patients in medical facilities with an unclear epidemiological status, returning to services like the AA, and the possible liberalization of abstinence and liver transplant rules in the COVID-19 era.
As our knowledge is still evolving, we hope there will be specific guidelines to answer these questions in the near future.
Conclusions
Patients with alcoholic liver disease and COVID-19 have worse prognosis. These patients are more likely to have an infection, including a COVID-19 infection. The clinical condition of both patients rapidly worsened and progressed to death; patient 1 had multi-organ failure and the patient 2 had cardiopulmonary insufficiency. Patients with pre-existing liver disease and COVID-19 are at increased risk for mortality as compared to patients without liver disease, and the relative risk was markedly higher in patients with cirrhosis. Alcoholic hepatitis increases the risk of a severe COVID-19 infection and a poor outcome. Due to frequent hospitalizations, patients are exposed to nosocomial infections, including the COVID-19 infection. To reduce the number of nosocomial infections, the standard recommendations and guidelines must be followed.
Figures
References:
1.. Guan WJ, Ni ZY, Hu Y, Clinical characteristics of 2019 novel coronavirus infection in China: N Engl J Med, 2020; 382(18); 1708-20
2.. Zhu N, Zhang D, Wang W, A novel coronavirus from patients with pneumonia in China, 2019: N Eng J Med, 2020; 282(8); 727-33
3.. Guo YR, Cao QD, Hong ZS, The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak– an update on the status: Mil Med Res, 2020; 7(1); 11
4.. Chen J, Qi T, Liu L, Clinical progression of patients with COVID-19 in Shanghai, China: J Infect, 2020; 80(5); e1-6
5.. Flisiak R, Horban A, Jaroszewicz J, Management of SARS-CoV-2 infection: Recommendations of the Polish Association of Epidemiologists and Infectologists as of March 31, 2020: Pol Arch Intern Med, 2020; 130; 352-57
6.. , Coronavirus disease (COVID-19) Situation Report – 209. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200816-covid-19-sitrep-209.pdf?sfvrsn=5dde1ca2_2
7.. Hamming I, Timens W, Bulthuis ML, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis: J Pathol, 2004; 203(2); 631-37
8.. Wong GL, Wong VW, Thompson A, Management of patients with liver derangement during the COVID-19 pandemic: An Asia-Pacific position statement: Lancet Gastroenterol Hepatol, 2020; 5(8); 776-87
9.. Sun J, Aghemo A, Forner A, Valenti L, COVID-19 and liver disease: Liv Int, 2020; 40(6); 1278-81
10.. Wang Y, Liu S, Liu H, SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19: J Hepatol, 2020 [Online ahead of print]
11.. Boettler T, Newsome PN, Mondelli MU, Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper: JHEP Rep, 2020; 2(3); 100113
12.. Kushner T, Cafardi J, Chronic liver disease and COVID-19: Alcohol use disorder/alcohol-associated liver disease, nonalcoholic liver disease/nonalcoholic steatohepatitis, autoimmune liver disease, and compensated cirrhosis: Clin Liver Dis, 2020; 15(5); 195-99
13.. Zhang C, Shi L, Wang FS, Liver injury in COVID-19: Management and challenges: Lancet Gastroenterol Hepatol, 2020; 5; 428-30
14.. Rickman HM, Rampling T, Shaw K, Nosocomial transmission of COVID-19: A retrospective study of 66 hospital-acquired cases in a London teaching hospital: Clin Infect Dis, 2020 [Online ahead of print]
15.. Zeair S, Cyprys S, Wiśniewska H, Alcohol relapse after liver transplantation: Younger women are at greatest risk: Ann Transplant, 2017; 22; 725-29
16.. Ranieri VM, Rubenfeld GD, Acute respiratory distress syndrome: The Berlin Definition: JAMA, 2012; 307(23); 2526-33
17.. Sofar AN, Barry V, Manos MM, The epidemiology and clinical characteristics of patients with newly diagnosed alcohol-related liver disease: results from population-based surveillance: J Clin Gastroenterol, 2010; 44(4); 301-7
18.. Bollipo S, Kapuria D, Rabiee A, One world, one pandemic, many guidelines: Management of liver disease during COVID-19: Gut, 2020; 69(8); 1369-72
19.. Levitsky J, Matillard ME, Diagnosis and therapy of alcoholic liver disease: Semin Liver Dis, 2004; 24(3); 233-47
20.. Maddrey WC, Boitnott JK, Bedine MS, Corticosteroid therapy of alcoholic hepatitis: Gastroenterology, 1978; 75(2); 193-99
21.. Arteel G, Marasano L, Mendez C, Advances in alcoholic liver disease: Best Pract Res Clin Gastroenterol, 2003; 17(3); 625-47
22.. Singh S, Khan A, Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in United States: A multicenter research network study: Gastroenterology, 2020; 159(2); 768-71
23.. Ridruejo E, Soza A, The liver in the times of COVID-19: What hepatologists should know: Ann Hepatol, 2020; 19(4); 353-58
24.. Simet SM, Sisson JH, Alcohol’s effects on lung health and immunity: Alcohol Res, 2015; 37; 199-208
25.. Da BL, Im GY, Schiano TD, COVID-19 Hangover: A rising tide of alcohol use disorder and alcohol-associated liver disease: Hepatology, 2020 [Online ahead of print]
26.. Mehta P, McAuley DF, Brown M, COVID-19: Consider cytokine storm syndromes and immunosuppression: Lancet, 2020; 395; 1033-34
27.. Xu L, Liu J, Lu M, Liver injury during highly pathogenic human corona-virus infections: Liver Int, 2020; 40(5); 998-1004
28.. Alhazzani W, Møller MH, Arabi YM, Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19): Intensive Care Med, 2020; 46(5); 854-87
29.. Thompson BT, Chambers RC, Liu KD, Acute respiratory distress syndrome: N Engl J Med, 2017; 377(6); 562-72
30.. Matthay MA, Zemans RL, Zimmerman GA, Acute respiratory distress syndrome: Nat Rev Dis Primers, 2019; 5(1); 18
31.. Goligher EC, Hodgson CL, Adhikari NK, Lung recruitment maneuvers for adult patients with acute respiratory distress syndrome: A systematic review and meta-analysis: Ann Am Thorac Soc, 2017; 14(Suppl. 4); S304-11
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250