10 June 2021: Articles 
An 80-Year-Old Woman with Alzheimer Disease and Accidental Poisoning with Pyrethroid Pesticide Successfully Treated with Intravenous Lipid Emulsion
Management of emergency care, Rare disease
Julio C. Diez-Sepulveda1ABCDEFG, Francisco L. Uribe-Buritica2ABCDEF*, Ana Maria Angel-Isaza1ABCDEF, Luis Alfonso Bustamante-Cristancho1BD, Felipe Mejia-Herrera1ABCDEF, Fredy A. Watts-Pajaro1ABCDEF, Maurix Fernando Rojas-Martinez3CDEDOI: 10.12659/AJCR.928420
Am J Case Rep 2021; 22:e928420
Abstract
BACKGROUND: Pesticides are commonly used in the agricultural industry. Overdose can be lethal due to its effects generating closure of the voltage-gated sodium channels in the axonal membranes. Most case reports of toxicity refer to skin exposure and there are very few that refer to effects due to its oral intake.
CASE REPORT: We report the case of an elderly woman with Alzheimer disease who accidentally swallowed 50 g of Lambda Cyhalothrin (GOLPE 5 M E®), a pyrethroid of medium toxicity containing a cyano group. It severely harmed the woman’s health, causing severe central nervous system depression and refractory vasodilated shock requiring the use of vasopressors. Its management was challenging, requiring orotracheal intubation, vasopressors, and admission to the Intensive Care Unit (ICU). The emergency care team decided to use intravenous lipid emulsion, which clearly helped with the recovery and successful discharge of the patient.
CONCLUSIONS: The use of intravenous lipid emulsion for the treatment of pyrethroid poisoning can lead to successful outcomes, as described in this case report.
Keywords: Emergency Service, Hospital, Fat Emulsions, Intravenous, Pyrethrins, Aged, 80 and over, Alzheimer Disease, Drug Overdose, Pesticides
Background
Due to an increase in agricultural use of pesticides, there have been growing concerns about the adverse effects on humans [1]. Studies have shown that acute and chronic exposure to pesticides has significant negative impacts on health that range from chronic health issues to death [1–3]. Pyrethroid pesticides have been used in agricultural and public health applications since 1970. Their mechanism of action is preventing the closure of the voltage-gated sodium channels in the axonal membranes, disrupting the nervous system, causing paralysis and death [4]. Few cases of systemic toxicity have been reported worldwide, but they can be life-threatening [5]. Signs and symptoms of pyrethroid poisoning depend on the type of pyrethroid and the amount of exposure. Type I pyrethroids (eg, bioallethrin, cismethrin, and permethrin) and type II pyrethroids (eg, cyhalothrin, cypermethrin, delta-methrin, and fenvalarate) are likely cause neurological symptoms. Type I pyrethroids usually generate severe fine tremors, marked reflex hyperexcitability, sympathetic activation, and paresthesias [6]. Type II pyrethroids cause different symptoms like profuse watery salivation, tremor, increased extensor tone, hyperexcitability, and activation of the sympathetic nervous system. They can also cause choreoathetosis, some types of seizures, and paresthesias. The toxic effects of pyre-throid exposure can be mild (eg, paresthesias, nausea, headache, vomiting, and fatigue), moderate (eg, CNS depression, increased salivation, fasciculations, fever, and diaphoresis), or severe (eg, seizures, coma, pulmonary edema, and respiratory failure) [7]. Pyrethroids have high selective toxicity to insects compared to mammals, which is due to higher nerve sensitivity in insects, lower mammalian skin absorption, and more efficient mammalian hepatic metabolism [8].
Case reports in the literature usually mostly involve skin exposure, which is the most common route of exposure. In this report, we present the case of an elderly woman with Alzheimer disease who swallowed a large quantity of pyrethroid pesticide. Management of the patient included lipid infusions and renal replacement therapy. She stayed several days in the Intensive Care Unit (ICU) and was discharged without major health consequences
Case Report
An 80-year-old woman with a medical history of hypothyroidism, high blood pressure, type 2 diabetes mellitus, and Alzheimer disease was admitted to the Emergency Department (ED) approximately 3 h after the ingestion of approximately 50 g of Lambda Cyhalothrin (GOLPE 5 M E®), a pyrethroid of medium toxicity containing a cyano group and an organic solvent. She was found by a family member, with a decreased level of consciousness, respiratory distress, and relaxation of sphincters. Therefore, the patient was taken to the ED.
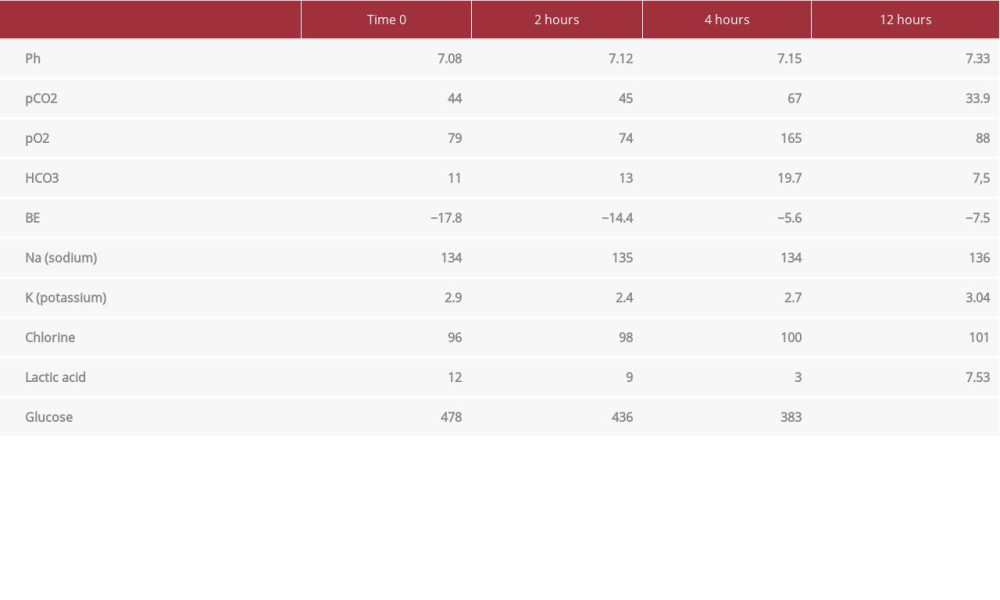
On admission to the ED, the patient had a Glasgow Coma Scale (GCS) of 13, heart rate 82 beats per min, blood pressure 167/92 mmHg, respiratory rate 26 breaths per min, oxygen saturation 89% with oxygen supplementation, and axillary temperature of 35.2°C. A physical examination revealed myoclonus and ballism in the upper limbs, mottled skin 2+, and mydriatic isochoric pupils. An admission ECG showed atrial fibrillation. Arterial blood gases showed mixed acidosis (Table 1). Her neurological status rapidly deteriorated 30 min after arrival at the ED, decreasing the GCS to 7, so orotracheal intubation was performed.
One hour after admission to the ED, she presented hemodynamic instability with sustained hypotension that did not respond to fluid management, requiring high doses of vasopressor (norepinephrine 0.9 mcg/kg/min).
Given the context of poisoning with a large amount of synthetic pyrethroid without a specific antidote available and the persistence of hemodynamic instability caused by a xenobiotic with some degree of liposolubility, was considered reasonable to start administering an intravenous lipid emulsion (ILE). We decided to administer SMOFlipid® at 20%, the ingredients of which include soybean oil, medium-chain triglycerides, refined olive oil, refined fish oil, omega 3 fatty acid, and egg lecithin. Intravenous perfusion of lipid emulsion was initiated at 1.5 ml/kg in bolus followed by a continuous infusion at 0.25 ml/kg/min. At the beginning of infusion of the lipid emulsion, the patient had a Glasgow Coma Scale score of 3 points, under sedation and orotracheal intubation, heart rate 108 beats per min, blood pressure 62/53 mmHg, respiratory rate 25 breaths per min, and 92% oxygen saturation with the following ventilatory parameters: positive end-expiratory pressure 8, 25 breaths per min, and 40% Fio2 (fraction of inspired oxygen). Two minutes after the ILE was started, her macrohemodynamics were: heart rate 111 beats per min, blood pressure 97/57 mmHg, respiratory rate 25 breaths per min, and 93% oxygen saturation.
Hemodialysis was initiated for severe and persistent metabolic acidemia associated with anuria 2 h after starting lipid emulsion therapy. Hemodialysis was performed over a period of 10 h.
In the ICU, she required vasoactive drugs with norepinephrine at 0.9 mcg/kg/min (60cc/h) and vasopressin 2.4 IU/h (14 cc/h). Twelve hours later, the vasoactive requirement decreased to norepinephrine 0.49 mcg/kg/min (30 cc/h), without vasopressin; a significant correction of metabolic acidosis was documented. Laboratory tests 12 h after admission to the ICU showed an improvement in the acid-base state, with gasometric control as follows: pH 7.33, pCO2 33.9, pO2 88, HCO3 7.5, and Be −7.4. Renal function also showed remarkable improvement, with ureic nitrogen of 14 and a creatinine of 0.1. Lactate remained elevated but this could have been influenced by other variables and was not necessarily due the state of hypoperfusion, and its value at this time was 7.53. Atrial fibrillation with rapid de novo ventricular response, without hemodynamic repercussions, was documented. It was managed with an amiodarone bolus of 300 mg and then 900 mg in continuous infusion for 24 h, returning to sinus rhythm. Subsequently, she presented clinical and paraclinical improvement, achieving extubation and removal of vasoactive agents on the 4th day of admission. She was transferred to the general ward, where she had a successful recovery.
Discussion
Emergency physicians commonly encounter multiple pathologies, including exogenous intoxications that can be life-threatening. Pesticide intoxication can be dangerous and lethal, and due to the many types of pesticides in use, its clinical presentation and management in the emergency room can be challenging. The present case report is of an uncommon route of intoxication and large exposure to a pesticide that is usually non-life-threatening. The patient swallowed the complete synthetic pyrethroid mixture, containing Lambda-cialotrina (50 g). The lethal dose in humans has not been determined, but in rats the oral median lethal dose (LD50) has been reported to be 144 mg/kg [9,10].
Systemic toxicity after pyrethroid exposure is rare. In cases of oral ingestion of large amounts, systemic toxicity can occur [11]. Our patient arrived at the ED with altered mental status and respiratory failure. Another important point is that the toxin ingested by our patient contains an organic solvent, but the chemical information sheet did not specify which one. It is of utmost importance to take this into account since they are not safe to ingest orally and may potentiate or synergize the toxic effects of the ingested substance [12]. With the use of pyrethroid in combination with volatile solvents, the symptoms due to poisoning can be worse due to solvent toxicity [13].
The effects of acute organic solvent poisoning have been well recognized for years; the symptoms consist of dizziness, light-headedness, and incoordination. Transient psychomotor impairment often accompanies such symptoms [14]. The management of skin exposure is to wash extensively until the pesticides are removed. Paresthesia can also be managed by washing the skin, and vitamin E can have some benefit in relieving this symptom [15,16]. If the pesticide was ingested, gastric lavage should be avoided because the solvents in the pyrethroid can increase the risk of aspiration pneumonia [11]. In cases of seizures and muscle fasciculation, the indicated treatment is diazepam (5-10 mg) [17].
Systemic poisoning is much difficult to manage. Two treatment approaches are used. The first is to antagonize some of the effects of the pyrethroid’s primary ion channel and thereby control some of the other effects mediated by neurotransmitter chemicals. Nevertheless, pyrethroids do not discriminate against a specific neurotransmitter system, and it has been demonstrated that both type I and type II pyrethroids can affect several neurotransmitter systems [18,19]. Type I and type II pyrethroids can be pro-convulsant through the GABA-ergic/ glutamatergic pathways [20,21]. However, the GABA antagonists are not useful in pyrethroid intoxication therapy. Also, the results of fewer doses of pyrethroids on inhibiting the hippocampus are not mediated by GABA [22]. Even the salivary feature of type II pyrethroid poisoning, although it is certainly cholinergic-mediated, is manageable by atropine in animals and humans [23,24]. It may also be very effective in controlling the chloride channel agonist ivermectin [25], which has no anticholinergic actions.
Thus, the best and most effective therapeutic method would be one that antagonizes sodium flow provoked by pyrethroids. Local anesthetics, anticonvulsants such as phenytoin, phenobarbitone, and valproate, and benzodiazepines are all ineffective [26].
It has been reported that mephenesin is helpful in type I pyrethroid poisoning only when the dose generates muscle relaxation. It also worked very effectively against type II poisoning at lower doses [27]. The long-term mephenesin derivative, methocarbamol, antagonizes motor effects of type I and type II pyrethroids [28].
There has also been proposed therapy through a voltage-dependent chloride channel site of action for type II pyrethroids (but not type I) [29]. Ivermectin acts as a chloride channel agonist and has been shown to be effective in reestablishing the vagus nerve membrane in rats who received an LD50 dose of deltamethrin. Ivermectin controls deltamethrin-induced salivation and repetitive muscle cramps [30]. Central nervous system actions of ivermectin can be limited by the multi-drug receptor pump on the blood-brain barrier, enabling ivermectin to reach the brain [31].
Due to persistent clinical deterioration with marked persistent hypotension that did not respond to vasoactives, an additional management strategy was considered, and the patient was started on intravenous lipid emulsion (ILE). ILE is a relatively new strategy to treat systemic toxicity due to local anesthetic overdose [32], first described in 1997, and experiments have demonstrated that rats pretreated with ILE became resistant to bupivacaine-induced cardiac arrest [33,34].
The use of lipid emulsion has been used for therapeutic complications of local anesthetics and acute drug poisoning. The first observation that lipid emulsions could sequester a lipid-soluble drug was reported in 1962, with a reduction in the duration of thiopental anesthetic in rats [35] and was described in 1974 in in vitro and in vivo studies with chlorpromazine and rabbit blood [36]. A search for improved therapy of acute bupivacaine toxicity led to experiments in rats in 1998, in which intravenous injection of a commercially available lipid emulsion improved recovery from bupivacaine-induced cardiotoxicity.
Several other experiments with animals followed [37,38] and the first human case reports of intravenous lipid emulsions as rescue or anti-toxic drug therapy were published in 2006 [37]. At our institution, we have the SMOFlipid® 20% brand lipid emulsion from the Fresenius Kabi laboratory. The lipid content of SMOFlipid is 0.20 g/mL and comprises a mixture of soybean oil, medium-chain triglycerides (MCTs), olive oil, and fish oil. The mean essential fatty acid content of SMOFlipid is supplied by Fresenius Kabi (Bad Homburg, Germany) and contains 35 mg/mL of linoleic acid (omega-6) (range, 28-50 mg/mL), 4.5 mg/mL of α-linolenic acid (omega-3) (range, 3-7 mg/mL), and a phosphate content of 15 mmol/L [39]. The total energy content of SMOFlipid from fat, glycerol, and phospholipids is 2000 Kcal/L [39]. Each 100 mL of SMOFlipid contains approximately 6 g soybean oil, 6 g MCT, 5 g olive oil, 3 g fish oil, 1.2 g egg phospholipids, 2.5 g glycerin, 16.3 to 22.5 mg all-rac-αtocopherol, 0.3 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9). SMOFlipid has an osmolality of approximately 380 mOsm/kg water (which represents an osmolarity of 270 mOsm/L). The oils included in SMOFlipid consist of a mixture of triglycerides, predominantly unsaturated fatty acids [39].
However, the current evidence supporting their use is unclear and the recommendations are inconsistent. To help physicians make decisions, the American Academy of Clinical Toxicology (ACMT) created a working group composed of international experts from various clinical specialties, including representatives from the major clinical toxicology associations. A rigorous methodology was developed using the Assessment of Research and Evaluation Guidelines and the AGREE II instrument to provide a framework for systematic reviews of this project and to make evidence-based recommendations on the use of ILE in poisoning [40].
Conclusions
The use of intravenous lipid emulsion in pyrethroid poisoning was successful in this case report. It may be an important alternative in the management of these patients. A small amount of evidence is available in the literature for the management of this type of poisoning.
References:
1.. Gyenwali D, Vaidya A, Tiwari S, Pesticide poisoning in Chitwan, Nepal: A descriptive epidemiological study: BMC Public Health, 2017; 17(1); 619
2.. Konradsen F, Acute pesticide poisoning – a global public health problem: Dan Med Bull, 2007; 54(1); 58-59
3.. Andersen HR, Debes F, Wohlfahrt-Veje C, Occupational pesticide exposure in early pregnancy associated with sex-specific neurobehavioral deficits in the children at school age: Neurotoxicol Teratol, 2015; 47; 1-9
4.. , Lambda-cyhalothrin: General Fact Sheet, 2001 http://npic.orst.edu/factsheets/l_cyhalogen.pdf
5.. Ray DE, Forshaw PJ, Poisoning syndromes, synergies, and therapy: J Toxicol Clin Toxicol, 2000; 38(2); 95-101
6.. Beasley M, Temple W, Typical clinical presentation of patients with pyrethroid exposure: Hazard Subst Ser, 2013(57); 41-43
7.. Litonius ES, Treatment of acute intoxication with intravenous lipid emulsion – animal and human studies, 2012, University of Helsinki Dissertation,
8.. Pennec JP, Guillouet M, Rannou F, Hemodynamic effects of lipid emulsion after local anesthetic intoxication may be due to a direct effect of fatty acids on myocardial voltage-dependent calcium channels: Can J Anesth, 2010; 57(10); 947
9.. Tomlin CDS: A World Compendium: The Pesticide Manual, 1997; 300-2, Farnham, Surrey, UK, British Crop Protection Council
10.. , Lambda cyhalothrin . [Accessed December 16, 2019http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/lambda-cyhalothrinext.html
11.. Bradberry SM, Cage SA, Proudfoot AT, Allister Vale J, Poisoning due to pyrethroids: Toxicol Rev, 2005; 24(2); 93-106
12.. Burgess WA, Recognition of health hazards in industry: John Wiley and Sons, 1981
13.. Ramchandra AM, Chacko B, Victor PJ, Pyrethroid poisoning: Indian J Crit Care Med, 2019; 23(Suppl. 4); S267-71
14.. Lessenger JE, Five office workers inadvertently exposed to cypermethrin: J Toxicol Environ Health, 1992; 35(4); 261-67
15.. Tucker SB, Flannigan SA, Cutaneous effects from occupational exposure to fenvalerate: Arch Toxicol, 1983; 54; 195-202
16.. Sasaki H, Nakamura J, Koh N, Effect of vitamin E and allylamine on the proliferation of cultured aortic smooth muscle cells from streptozotocin-induced diabetic rats: Life Sci, 1999; 64(25); 2317-25
17.. Flannigan SA, Tucker SB, Ross CE, Synthetic pyrethroid insecticides: A dermatological evaluation: Br J Ind Med, 1985; 42(6); 363-72
18.. Gammon DW, Lawrence LJ, Casida JE, Pyrethroid toxicology: Protective effects of diazepam and phenobarbital in the mouse and the cockroach: Toxicol Appl Pharmacol, 1982; 66(2); 290-96
19.. Staatz CG, Bloom AS LJ, A pharmacological study of pyrethroid neurotoxicity in mice: Biochem Physiol, 1982; 17; 287-92
20.. Gammon DW, Sander G, Two mechanisms of pyrethroid action: electrophysiological and pharmacological evidence: Neurotoxicology, 1983; 6; 63-85
21.. Chanh PH, Navarro-Delmasure C, Chanh APH, Pharmacological effects of deltamethrin on the central nervous system: Drug Res, 1984; 34; 175-81
22.. Devaud LL, Szot P, Murray T, PK 11195 antagonism of pyrethroid-induced proconvulsant activity: Eur J Pharmacol, 1986; 121; 269-73
23.. Joy RM, Albertson TE, Interactions of GABA-A antagonists with deltamethrin, diazepam, pentobarbital, and skf100330a in the rat dentate gyrus: Toxicol Appl Pharmacol, 1991; 109; 251-62
24.. Ray DE, Cremer JE, The action of decamethrin (a synthetic pyrethroid) on the rat: Pestic Biochem Physiol, 1979; 10; 333-40
25.. He F, Wang S, Liu L, Clinical manifestations and diagnosis of acute pyrethroid poisoning: Arch Toxicol, 1989; 63(1); 54-58
26.. Oortgiesen M, van Kleef DM, Block of deltamethrin-modified sodium current in cultured mouse neuroblastoma cells: Local anaesthetics as potential antidotes: Brain Res, 1990; 518; 11-18
27.. Bradbury JE, Forshaw PJ, Gray AJ, The action of mephenesin and other agents on the effects produced by 2 neurotoxic pyrethroids in the intact and spinal rat: Neuropharmacology, 1983; 22; 907-14
28.. Hiromori T, Nakanishi T, Kawaguchi S, Therapeutic effects of methocarbarnol on acute intoxication by pyrethroids in rats: J Pestic Sci, 1986; 11; 9-14
29.. Ray DE, Sutharsan S, Actions of pyrethroid insecticides on voltage-gated chlo-ride channels in neuroblastoma cells: Neurotoxicol Teratol, 1997; 18; 755-60
30.. Forshaw PJ, A novel action of deltamethrin on membrane resistance in mammalian skeletal muscle and nonmyelinated nerve fibers: Neuropharmacology, 1990; 29; 75-81
31.. Schinkel AH, Wagenaar E, Mol AM, Pglycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs: J Clin Invest, 1996; 97; 2517-24
32.. LeClercq M, Cotonat J FP, [Search for an antagonist for deltamethrin intoxication]: J Toxicol Clin Exper, 1986; 6; 85-93 [in French]
33.. Rothschild L, Bern S, Oswald S, Weinberg G, Intravenous lipid emulsion in clinical toxicology: Scand J Trauma Resusc Emerg Med, 2010; 18(1); 1-8
34.. Weinberg GL, Intravenous lipid emulsion: Why wait to save a life?: Emerg Med Australas, 2011; 23(2); 113-15
35.. Russell R, Alleviation of barbiturate depression by fat emulsion: Anesth Analg, 1962; 41; 582-85
36.. Krieglestein J, Meffert A, Influence of emulsified fat on chlorpromazine availability in rabbit blood: Experientia, 1974; 30; 924-26
37.. Jamaty C, Bailey B, Larocque A, A systematic review of human and animal studies: Clin Toxicol (Phila), 2010; 48(1); 1-27
38.. Cave G, Harvey M, Intravenous lipid emulsion as antidote beyond local anesthetic toxicity: A systematic review: Acad Emerg Med, 2009; 16(9); 815-24
39.. Kabi Fresenius, California USA, Product Information: Smoflipid Jan, 2021 https://medlibrary.org/lib/rx/meds/smoflipid/page/4/
40.. Gosselin S, Hoegberg LC, Hoffman RS, Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning: Clin Toxicol (Phila), 2016; 54(10); 899-923
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250