01 May 2021: Articles 
Aggressive Resection of Malignant Paraaortic and Pelvic Tumors Accompanied by Arterial Reconstruction with Synthetic Arterial Graft
Unusual setting of medical care
Ryotaro Tani1BDF, Tomohide Hori1ABCDEF*, Hidekazu Yamamoto1DF, Hideki Harada1DF, Michihiro Yamamoto1DF, Masahiro Yamada1DF, Takefumi Yazawa1DF, Ben Sasaki1DF, Masaki Tani1DF, Asahi Sato1DF, Hikotaro Katsura1DF, Yasuyuki Kamada1DF, Ryuhei Aoyama1DF, Yudai Sasaki1DF, Masazumi Zaima1ADFDOI: 10.12659/AJCR.931569
Am J Case Rep 2021; 22:e931569
Abstract
BACKGROUND: Advanced malignancies in the lower abdomen easily invade the retroperitoneal and pelvic space and often metastasize to the paraaortic and pelvic lymph nodes (LNs), resulting in paraaortic and/or pelvic tumor (PPT).
CASE REPORT: A total of 7 cases of aggressive malignant PPT resection and orthotopic replacement of the abdominal aorta and/or iliac arteries with synthetic arterial graft (SAG) were experienced during 16 years. We present our experience with aggressive resection of malignant PPTs accompanied by arterial reconstruction with SAG in detail. The primary diseases included 2 cases endometrial cancer and 2 cases of rectal cancer, and 1 case each of ovarian carcinosarcoma, vaginal malignant melanoma, and sigmoid cancer. Surgical procedures are described in detail. Briefly, the abdominal aorta and iliac arteries were anastomosed to the SAG by continuous running suture using unabsorbent polypropylene. Five Y-shaped and 2 I-shaped SAGs were used. This en bloc resection actually provided safe surgical margins, and tumor exposures were not pathologically observed in the cut surfaces. Graphical and surgical curability were obtained in all cases in which aggressive malignant PPT resections were performed. The short-term postoperative course of our patients was uneventful. From a vascular perspective, the SAGs remained patent over the long term after surgery, and long-term oncologic outcomes were satisfactory.
CONCLUSIONS: To our knowledge, this case series is the first report of aggressive malignant PPT resection accompanied by arterial reconstruction with SAG. This procedure is safe and feasible, shows curative potential, and may play a role in multidisciplinary management of malignant PPTs.
Keywords: Arteries, Neoplasms, Prostheses and Implants, Iliac Artery, Pelvic Neoplasms, Reconstructive Surgical Procedures, Vascular Surgical Procedures
Background
Advanced malignancies in the lower abdomen (eg, colorectal, urological, and gynecological neoplasms) easily invade the retroperitoneal and pelvic space and often metastasize to the paraaortic and pelvic lymph nodes (LNs), resulting in paraaortic and/or pelvic tumors (PPT) [1–4]. Even after radical surgery, local recurrence and/or LN metastasis can cause PPT formation [4,5]. We present our experience with aggressive malignant PPT resection and orthotopic replacement of the abdominal aorta and/or iliac arteries with a synthetic arterial graft (SAG), which has not been previously reported, and discuss treatment of locally invasive, metastatic, and recurrent PPTs.
Case Reports
CASE 1:
A 53-year-old woman diagnosed with T3N0M0 stage IIIC ovarian carcinosarcoma underwent neoadjuvant chemotherapy followed by non-curative unilateral resection of the appendicular organ, total omentectomy, and adjuvant chemotherapy. Metachronous recurrence of PPT occurred 1.8 years after the initial surgery. Aggressive tumor resection accompanied by arterial reconstruction with SAG and inferior vena cava (IVC) resection was then performed. An I-shaped SAG was used to replace the abdominal aorta from the lower level of the renal artery (RA) to the upper level of the inferior mesenteric artery (IMA) (Figure 2A). The IVC and left renal vein were resected, but venous reconstruction was not performed. Venous flow into the IVC was kept via developed collaterals in the pelvic space, gluteus maximus muscle, mesorectum and mesocolon, retroperitoneal space around the iliopsoas muscle, and Gerota fascia (Figure 3A–3E). These developed collaterals from the IVC flowed into the inferior and superior mesenteric veins and splenic vein (Figure 3A–3E). Venous flow of the left renal vein was kept mainly via splenorenal shunt, and other developed collaterals from the left renal vein flowed into the superior mesenteric vein and IVC (Figure 3A–3E). Congestion or flow disorder was not observed in the left kidney. Hence, venous flow from the IVC and left renal vein were well preserved by developed collaterals and splenorenal shunt from the early postoperative period. Lung and mediastinal LN metastases were detected 1.3 years later and she died 5.9 years after diagnosis.
CASE 2:
A 70-year-old woman diagnosed with T1bN1M0 stage IIIC endometrial cancer of the uterus with PPT underwent extended total hysterectomy, bilateral resection of the appendicular organs, paraaortic and pelvic lymphadenectomy, and partial resection of the IVC. A Y-shaped SAG was used to replace the aorta from the root of the IMA to the common iliac arteries (CIAs) 1 cm distal to the bifurcation bilaterally (Figure 2B). The patency of the partially resected IVC was well preserved from the early postoperative period (Figure 3F). Adjuvant chemo-therapy was administered after surgery. Mediastinal LN metastases were detected 1.6 years after surgery and she remained alive at 9.0 years after diagnosis.
CASE 3:
A 53-year-old woman diagnosed with T4bN0M0 stage IVA vaginal malignant melanoma underwent partial resection of the vaginal wall and postoperative chemotherapy. Subsequent local recurrences at the urethral orifice and vaginal wall and metastatic inguinal LN were resected. PPT was detected 2.6 years after the initial surgery and removed
CASE 4:
A 48-year-old woman diagnosed with T3bN2M0 stage IIIC endometrial cancer of the uterus with PPT underwent total abdominal hysterectomy, bilateral resection of the appendicular organs, and paraaortic and pelvic lymphadenectomy. The PPT was removed in en bloc and a Y-shaped SAG was used to replace the aorta from the lower level of the RA to the CIAs distal to the bifurcation bilaterally. Graphical and surgical curability was obtained (Figure 4A, 4B). Adjuvant chemotherapy was administered after surgery. Peritoneal dissemination was detected 0.8 years after surgery and she remains alive 2.1 years after diagnosis.
CASE 5:
A 66-year-old man diagnosed with T3N2bM0 stage IIIC moderately-differentiated adenocarcinoma of the rectum underwent anterior resection. After paraaortic LN metastases were detected 1.4 years later, they were resected and chemotherapy was introduced. PPT was detected 4.0 years after surgery and aggressive resection accompanied by arterial reconstruction with SAG was performed. An I-shaped graft was used to replace the aorta from the lower level of the RA to the lower level of the IMA. Lung and mediastinal LN metastases were detected 0.3 years after PPT resection and chemotherapy was resumed. He died 4.8 years after diagnosis.
CASE 6:
A 43-year-old woman diagnosed with T3N2aM0 stage IIIB well-differentiated adenocarcinoma of the sigmoid colon underwent radical resection with lymphadenectomy. After paraaortic LN metastases were detected 0.6 years later and removed, chemotherapy was introduced. PPT was detected 4.0 years after the initial surgery and aggressive resection accompanied by arterial reconstruction with SAG and IVC resection was performed. A Y-shaped graft was used to replace the aorta from the lower level of the RA to the CIAs distal to the bifurcation bilaterally and adjuvant chemotherapy was resumed. Lung and mediastinal LN metastases were detected 0.3 years after PPT resection. She died 3.2 years after diagnosis.
CASE 7:
A 76-year-old man diagnosed with T3N0M0 stage IIA well-differentiated adenocarcinoma of the rectum underwent low anterior resection with lymphadenectomy. PPT was detected 1.7 years after surgery and aggressive resection accompanied by arterial reconstruction with SAG and left ureter re-section was performed. A Y-shaped graft was used to replace the aorta from the lower level of the RA to the CIAs distal to the bifurcation bilaterally. The ureter was reconstructed with an end-to-end anastomosis. Graphical and surgical curability was obtained (Figure 4C, 4D). Image studies by dynamic computed tomography and 18F-fluorodeoxyglucose positron emission tomography were repeated every 6 months after en bloc resection of the PPT. Peritoneal dissemination and lung metastasis were detected 2.9 years after PPT resection and chemotherapy was introduced. He died 9.2 years after diagnosis.
Discussion
Advanced malignancies in the lower abdomen can easily invade regional structures, metastasize to local and regional LNs, and disseminate throughout the intraperitoneal space [3,4]. Although intentional removal of local recurrence and extended lymphadenectomy of paraaortic and/or pelvic LNs may have oncologic therapeutic potential for some malignancies [1-5], extended lymphadenectomy is clearly contraindicated in some diseases [8,9]. Therefore, few cases of aggressive resection for malignant PPTs have been reported [10–12]. Arterial reconstruction was not performed in these cases; however, arterial bypass (ie, heterotopic replacement) via SAG has been described [11,12]. To our knowledge, our case series is the first report of aggressive PPT resection accompanied by arterial reconstruction (ie, orthotopic replacement) with SAG.
Pathological examination in our case series revealed that direct invasion of the arterial wall may not be observed in some cases, even when nervous plexuses, lymphatic ducts, and surrounding vessels are clearly invaded. In all of our cases, the PPT was removed
Details of our surgical procedures are shown in Table 2 and Figure 2. The short-term postoperative course in our patients was uneventful (Tables 2, 3). From a vascular perspective, the SAGs remained patent over the long term after surgery (Table 3) and long-term oncologic outcomes were satisfactory (Table 3, Figure 1), suggesting that our results are acceptable. Multidisciplinary therapy is crucial for advanced cancers [13]. Aggressive resection accompanied by arterial reconstruction with SAG may have a role in multidisciplinary treatment of malignant PPTs.
To our knowledge, this case series is the first report of aggressive malignant PPT resection accompanied by arterial reconstruction with SAG. This procedure is safe and feasible, shows curative potential, and may play a role in multidisciplinary management of malignant PPTs. Surgical indications for aggressive resection of malignant PPTs accompanied by arterial reconstruction (ie, orthotopic replacement) with SAG should be carefully decided on a case-by-case basis. This
Conclusions
We reported our experience with aggressive resection of malignant PPTs accompanied by arterial reconstruction (ie, ortho-topic replacement) with SAG. Our results suggest that this approach is safe and feasible, with satisfactory outcomes.
Figures
References:
1.. Kanemitsu Y, Shida D, Tsukamoto S, Japanese evidences on nervepreserving lateral pelvic lymh node dissection for rectal cancer: Major historical milestones and clinical impact: The past, present and future: Clin Colon Rectal Surg, 2020; 33; 349-54
2.. Kanno T, Kobori G, Kubota M, Standardized and simplified retroperitoneal lymph node dissection during retroperitoneal laparoscopic radical nephroureterectomy for urothelial carcinoma of the upper ureter or renal pelvis: en bloc resection technique: Urology, 2018; 112; 85-91
3.. Zanfagnin V, Huang Y, Mc Gree ME, Predictors of extensive lymphatic dissemination and recurrences in node-positive endometrial cancer: Gynecol Oncol, 2019; 154; 480-86
4.. Zhou J, Shan G, Chen Y, The effect of lymphadenectomy on survival and recurrence in patients with ovarian cancer: A systematic review and meta-analysis: Jpn J Clin Oncol, 2016; 46; 718-26
5.. Uehara K, Ito Z, Yoshino Y, Aggressive surgical treatment with bony pelvic resection for locally recurrent rectal cancer: Eur J Surg Oncol, 2015; 41; 413-20
6.. : TNM classification of malignant tumours, 2017, New York, Willey Blackwell
7.. Slankamenac K, Graf R, Barkun J, The comprehensive complication index: A novel continuous scale to measure surgical morbidity: Ann Surg, 2013; 258; 1-7
8.. Tol JA, Gouma DJ, Bassi C, Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the International Study Group on Pancreatic Surgery (ISGPS): Surgery, 2014; 156; 591-600
9.. Sasako M, Sano T, Yamamoto S, D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer: N Engl J Med, 2008; 359; 453-62
10.. Sueda T, Noura S, Ohue M, [A case of rectal cancer with long-term disease-free survival following resection of the right iliac artery due to isolated para-aortic lymph node recurrence.]: Gan To Kagaku Ryoho, 2012; 39; 2258-60 [in Japanese]
11.. Abdelsattar ZM, Mathis KL, Colibaseanu DT, Surgery for locally advanced recurrent colorectal cancer involving the aortoiliac axis: can we achieve R0 resection and long-term survival?: Dis Colon Rectum, 2013; 56; 711-16
12.. Takaichi S, Morimoto Y, Fujii H, [Resection od advanced cecum cancer invading the right external iliac artery and vein preceded by vascular reconstruction – report of a case.]: Journal of Japan Surgical Association, 2017; 78; 1312-17 [in Japanese]
13.. Alawawdeh A, Krishnan T, Roy A, Curative therapy for rectal cancer: Expert Rev Anticancer Ther, 2021; 21(2); 193-203
Figures
Tables
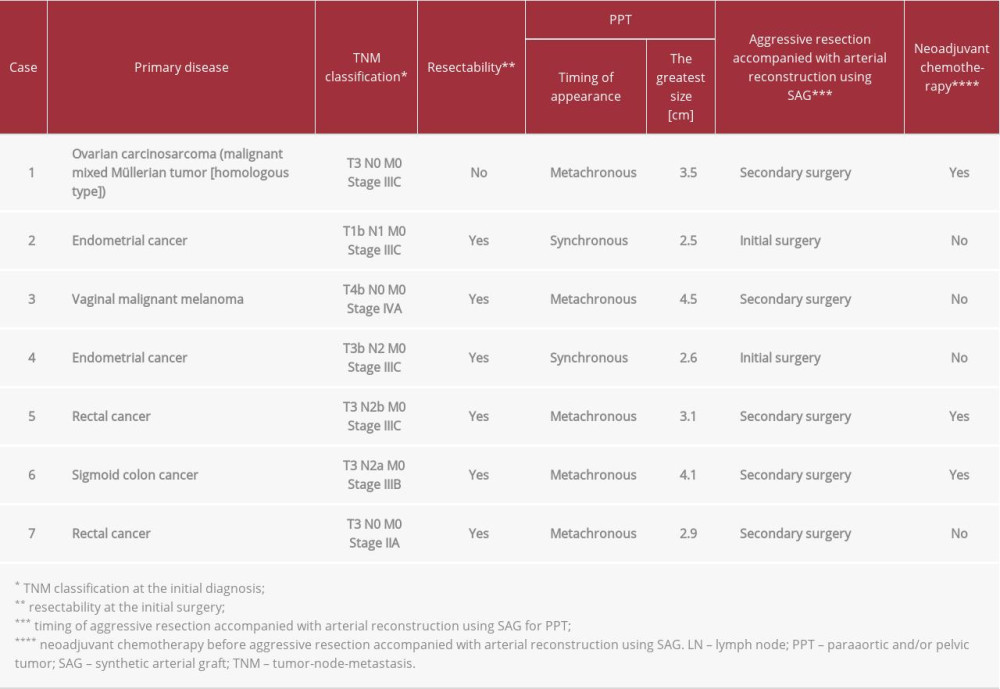 Table 1.. Patients’ profiles.
Table 1.. Patients’ profiles.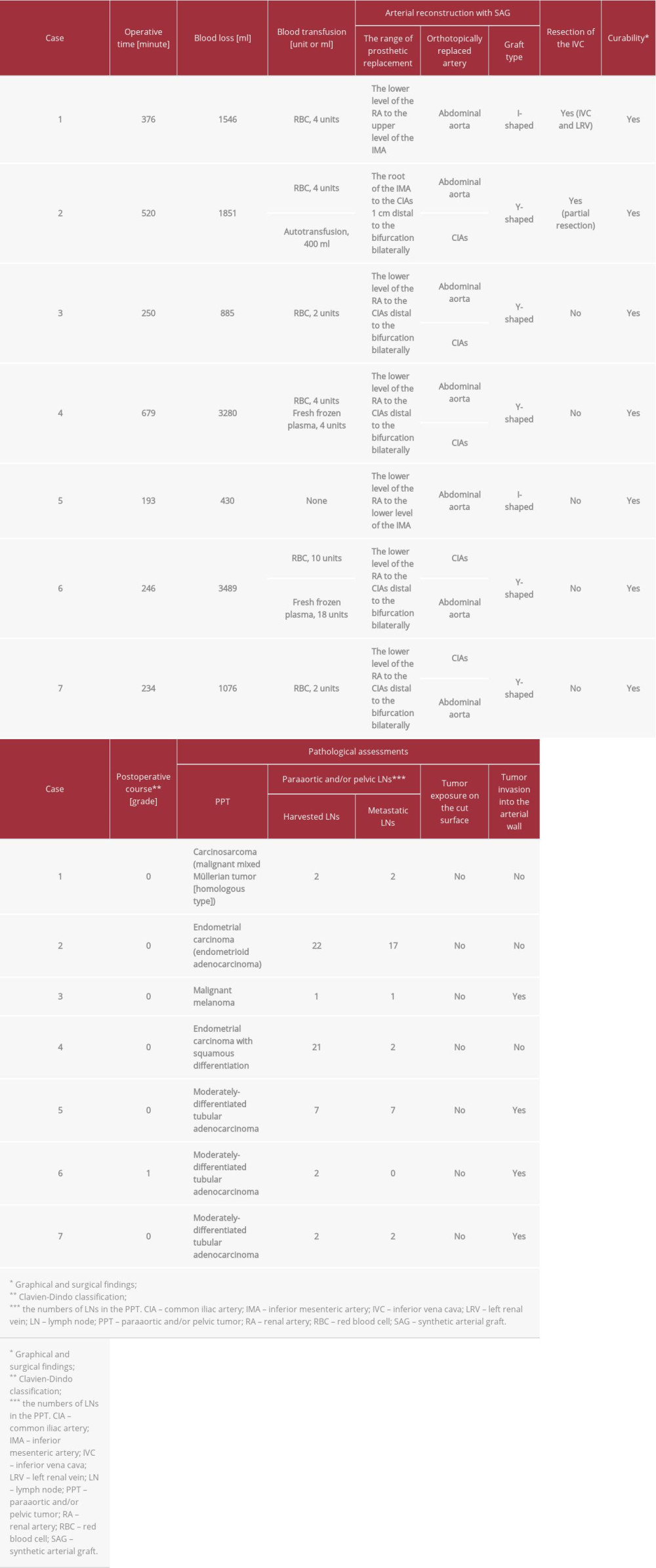 Table 2.. Intraoperative findings and pathological assessments of PPTs.
Table 2.. Intraoperative findings and pathological assessments of PPTs.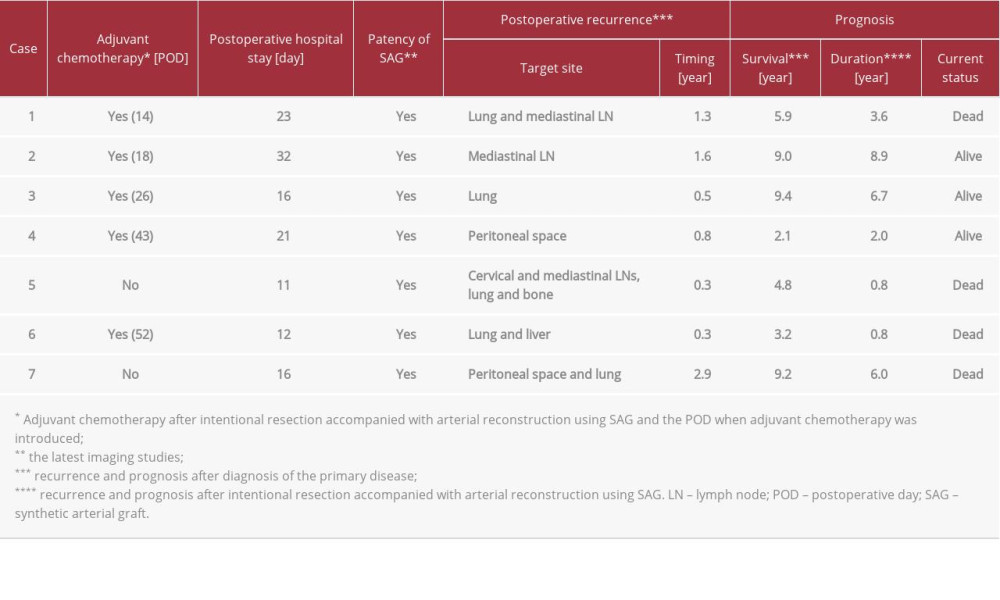 Table 3.. Postoperative courses and prognostic outcomes after aggressive resection accompanied with arterial reconstruction using SAG.
Table 3.. Postoperative courses and prognostic outcomes after aggressive resection accompanied with arterial reconstruction using SAG. Table 1.. Patients’ profiles.
Table 1.. Patients’ profiles. Table 2.. Intraoperative findings and pathological assessments of PPTs.
Table 2.. Intraoperative findings and pathological assessments of PPTs. Table 3.. Postoperative courses and prognostic outcomes after aggressive resection accompanied with arterial reconstruction using SAG.
Table 3.. Postoperative courses and prognostic outcomes after aggressive resection accompanied with arterial reconstruction using SAG. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








