03 July 2021: Articles 
Thrombocytopenia Induced by Polysulfone Dialysis Membranes
Unusual or unexpected effect of treatment
Ivan Claudio-Gonzalez1BCDEF, Deepak Ravindranathan2BDE, Christine L. KemptonDOI: 10.12659/AJCR.932045
Am J Case Rep 2021; 22:e932045
Abstract
BACKGROUND: Biocompatible hemodialysis membranes have greatly advanced the treatment of renal failure. Synthetic polysulfone dialysis membranes are considered to be very biocompatible because of their low propensity to activate complement. However, these membranes can reduce platelet count through platelet activation, although the mechanism of this activation is unknown.
CASE REPORT: We report the case of an 82-year-old man with a history of chronic kidney disease with recurrent gastrointestinal bleeding and worsening renal function who was initiated on renal replacement therapy with polysulfone dialysis membranes. On admission, the patient’s platelet count was normal at 233×10³/μL. A significant fall in platelet count was observed following most dialysis treatments, reaching a nadir of 37×10³/μL. With occasional dialysis treatments, his platelet count did not change. This dialysis-induced thrombocytopenia resolved following substitution with Cellentia-H cellulose triacetate single-use, hollow-fiber, high-flux hemodialyzer membrane.
CONCLUSIONS: Polysulfone membranes are capable of activating platelets, which can result in severe thrombocytopenia. However, the magnitude of dialysis-induced thrombocytopenia varies from treatment to treatment. As such, it may not be evident when the pre- and postdialysis platelet counts are measured for a single treatment. Because the etiology of this platelet activation is unknown, substitution with cellulose triacetate membranes should be considered. These membranes have an unrelated chemical composition and a very low propensity to activate platelets.
Keywords: Kidneys, Artificial, Platelet Activation, Thrombocytopenia, Aged, 80 and over, Biocompatible Materials, Membranes, Artificial, Polymers, Renal Dialysis, Sulfones
Background
Dialysis-associated thrombocytopenia is a rare but important complication of renal replacement therapy [1]. A dialysis membrane’s biocompatibility is determined by the extent to which it changes the white blood cell count or activates complement [2]. The dialysis-induced thrombocytopenia seen with older cuprophane membranes was attributed to complement activation [3]. Newer products, such as polysulfone membranes, are thought to be very biocompatible because they produce little complement activation [4]. Nevertheless, polysulfone membranes can activate platelets and thereby reduce total platelet count, although such treatments generally reduce platelet count by less than 5% [5]. We report the case of a patient with a normal platelet count at baseline, who experienced a marked decline in platelet count following dialysis with polysulfone membranes, which resolved with cellulose triacetate membrane substitution. Because the etiology of this polysulfone membrane-induced fall in platelet count is unclear, how it should be treated and which membrane or membranes should be substituted are not established. We suggest that substituting these polysulfone membranes with cellulose triacetate is a reasonable first step in treating this disorder.
Case Report
An 82-year-old man with a history of hypertension, an abdominal aortic aneurysm, stage 5 chronic kidney disease, and duodenal and jejunal arteriovenous malformations (AVMs) was admitted to Emory University Hospital with recurrent gastrointestinal bleeding and acute kidney injury. One year prior to admission, the patient experienced a cecal perforation following a colonoscopy, which was repaired with a right hemicolectomy. His physical examination was unremarkable. Admission laboratory values are reported in Table 1. Given his low hemoglobin, he received red blood cell transfusions to a threshold of 7 g/dL.
A computed tomography angiogram of the abdomen and pelvis showed an arterial blush with persistence on the venous phase in the distal ascending proximal transverse colon around the surgical anastomosis. A colonoscopy showed AVMs that were clipped, with resolution of the bleeding. His hemoglobin thereafter remained stable at 8.5 g/dL. After obtaining informed, written consent, dialysis was initiated using a Permacath™ 14.5-Fr Dual Lumen Catheter for vascular access and F180NR Optiflux polysulfone hemodialysis membranes (Fresenius Medical Care, Waltham, MA).
Figure 1 shows the relationship between dialysis treatments and the patient’s platelet count. His predialysis platelet count was 233×103/μL, which fell to 81×103/μL measured 3 h after termination of this first treatment. This dialysis-associated fall in platelet count was not associated with fever, chills, shortness of breath, or hypotension while on dialysis. The following day he was again dialyzed with pre- and postdialysis platelet counts of 100×103/μL and 62×103/μL, respectively. Over the ensuing 5 days he received no dialysis treatments, whereupon his platelet count rose from 62×103/μL to 216×103/μL. When dialysis was resumed, his platelet count fell by ~40% with most dialyses, causing a progressive decline in platelet count to a nadir of 37×103/μL on hospital day 38. While the patient’s platelet count generally rose in the interval between dialyses, this response was variable. By linear regression analysis, his platelet count rose by ~(20–30)×103 cells/mL/d following the dialyses on hospital days 9, 15, 26, and 33. However, his platelet count did not increase following the treatments on hospital days 19 and 22.
The patient was dialyzed with citrate anticoagulation and did not receive heparin. New medications started during this hospitalization were limited to midodrine and amlodipine. Rinsing the membrane with 1 L of normal saline prior to the dialysis treatment did not improve the dialysis-induced thrombocytopenia.
Hematology was consulted for evaluation of thrombocytopenia, and the results of their workup are displayed in Table 1. This evaluation revealed no splenomegaly on physical examination or schistocytes or platelet clumping on peripheral smear. The activated partial thromboplastin time (aPTT) was normal throughout the hospitalization. His mildly elevated prothrombin time was attributed to the liver disease detected by ultrasound since he had not taken vitamin K antagonists. Disseminated intravascular coagulation (DIC) was considered owing to the increasing d-dimer and declining fibrinogen level. However, since the aPTT was not elevated and since a score of 4 points was calculated based on the International Society of Thrombosis and Haemostasis DIC criteria, acute DIC seemed unlikely. Instead, the elevated d-dimer was attributed to rectal bleeding from colonic AVMs detected by colonoscopy. Given his history of gastrointestinal bleeding from AVMs, acquired von Willebrand disease was suspected but ruled out with a normal von Willebrand factor antigen level and elevated ristocetin cofactor activity, the latter of which was attributed to inflammation. Anti-heparin platelet factor 4 antibody titer levels did not meet the cutoff for heparin-induced thrombocytopenia. Vitamin B12 level was normal. Folate was initially low but normalized with supplementation. HIV and hepatitis B tests were negative.
Polysulfone dialysis membrane-induced thrombocytopenia was suspected due to the strong correlation between dialysis treatments and the patient’s platelet count decline. The dialysis membrane was subsequently changed to a Cellentia-17H cellulose triacetate single-use, hollow-fiber, high-flux hemodialyzer (Nipro Medical Corporation, Bridgewater, NJ) starting on hospital day 40. The patient received 3 dialysis treatments over the next week with this new membrane, over which time his platelet count rose to 120×103/μL with resolution of the dialysis-induced fall in platelet count. After discharge, the patient continued to receive outpatient hemodialysis 3 times weekly with cellulose triacetate dialysis membranes. His platelet count was 175×103/μL measured 22 days after discharge.
Discussion
Thrombocytopenia is common in hospitalized patients. Among critically ill patients, 46.5% have at least 1 documented platelet count of less than 150×103/μL, while 23.9% have persistent thrombocytopenia [6]. Thrombocytopenia can occur from platelet underproduction, increased destruction of platelets, or platelet sequestration. Platelet underproduction can result from bone marrow processes, liver failure, and medications. Platelet destruction can occur with immune thrombocytopenic purpura, thrombotic thrombocytopenic purpura, or disseminated intravascular coagulation. None of these entities were seen in this patient.
We suspected dialyzer membrane-associated thrombocytopenia in our patient because of the strong correlation between changes in platelet count and dialysis treatments and through the elimination of other potential causes. Our patient, as well as patients in previous reports, showed variable recovery in platelet count between dialysis treatments. Some previous reports of polysulfone membrane-induced thrombocytopenia observed a return to baseline levels in platelet count in the interdialytic period [4], while others did not [7]. Our case report describes in detail the relationship between dialysis treatments with polysulfone membranes and platelet count in a patient over his first 30 days of dialysis. This case shows for the first time that the platelet count response to these membranes can be highly variable. Our patient’s platelet count fell dramatically with some treatments and then recovered to normal baseline levels, while with other sessions there was little change in platelet count either before and after dialysis or within the period between dialyses. As such, it may be difficult to appreciate polysulfone membrane-induced thrombocytopenia when the relationship between platelet count and dialysis is examined over a short time frame, particularly once polysulfone membrane-induced thrombocytopenia is established. Therefore, polysulfone membrane-induced thrombocytopenia should be considered in thrombocytopenic dialysis patients even in the absence of a consistent change in platelet count with these treatments.
A dialysis-induced fall in platelet count is a well-known complication of renal replacement therapy, although this decline is generally small. Typically, platelet counts fall during the first hour of dialysis and then return to baseline values by the end of the treatment [5]. However, isolated case reports observed more than a 50% decline in platelet count following hemo-dialysis with polysulfone membranes, which resulted in significant thrombocytopenia with platelet counts falling below 100×103/μL [7]. While the thrombocytopenia observed with older cuprophane membranes was attributed to complement activation, polysulfone membranes produce little complement response [7].
The fall in platelet count observed following intermittent hemodialysis with polysulfone membranes is generally less than 5% [5]. However, Liu et al [8] observed platelet count to be ~20% lower 12 h after initiation of continuous veno-venous hemofiltration (CVVH) with polysulfone membranes relative to pretreatment levels. Risk factors for the dialysis-induced thrombocytopenia observed with electron beam-sterilized polysulfone membranes include advanced age, primary kidney disease, and lower predialysis platelet and white counts [9]. Of these, our patient’s only risk factor was advanced age.
The mechanism responsible for polysulfone-induced thrombocytopenia is unknown. The fall in platelet count seen with polysulfone membranes has been attributed to platelet activation, rather than complement activation, with an ensuing increase in thrombin-antithrombin complexes, which increases platelet consumption [7]. The platelet consumption seen with these membranes has been attributed to the electron beam sterilization process [9], subtle differences in membrane geometry or composition that exist among dialyzers from various manufacturers [5], endotoxins introduced during dialysis, or idiosyncratic reactions [10]. This thrombocytopenia is seen less often with dialyzer reuse [11]. Some [7,12], but not all [13], reported cases of Fresinius Optiflux Polysulfone membrane-associated thrombocytopenia resolved with the use of polysulfone membranes not sterilized with an electron beam. Nonetheless, a study of 1706 Canadian hemodialysis patients revealed a strong correlation between electron beam sterilization and dialysis-induced thrombocytopenia [9]. Moreover, the polysulfone membrane-induced thrombocytopenia resolved in some patients [7], but not all [13], with substitution of a polysulfone membrane with slightly different chemical composition from another manufacturer. As such, the fall in platelet count that can occur with the use of polysulfone membranes does not always respond to substitution with a polysulfone membrane with a slightly different chemical composition from another manufacturer or one that has not been sterilized with an electron beam.
Because polysulfone membrane-induced thrombocytopenia likely has many causes, how it should be treated is unclear. Case reports show more consistent resolution of the thrombocytopenia, however, when the substituting dialyzer is not composed of polysulfone. These reports have observed resolution of polysulfone membrane-induced thrombocytopenia following substitution with a Baxter cellulose triacetate excel-tra 210 membrane [4]; an alkyl ether polymer-grafted cellulose membrane [13]; or an ethylene vinyl alcohol, polymethyl methacrylate, or polyester polymer alloy membrane [14]. We conclude that resolution of the polysulfone membrane-induced thrombocytopenia is more likely when the membrane is substituted with one of completely different composition that is not electron beam sterilized.
Platelet activation and the subsequent fall in platelet count are less common with cellulose than with polysulfone dialysis membranes [4]. Liu et al [8] measured platelet activation and platelet count in 96 Intensive Care Unit patients receiving CVVH. While these authors observed platelet activation and an 18% fall in platelet count in the patients undergoing CVVH with polysulfone dialyzers, they did not see platelet activation or a change in platelet count in those receiving CVVH with cellulose triacetate membranes. Thus, dialysis-induced thrombocytopenia is absent or very rare with cellulose triacetate membranes.
We conclude that once polysulfone membrane-induced thrombocytopenia is recognized, substitution with a cellulose triacetate dialyzer is a good first option. First, platelet activation is much lower with this membrane class. Second, cellulose tri-acetate membranes are sterilized without an electron beam. Third, cellulose triacetate and polysulfone membranes are chemically unrelated, and fourth, cellulose membranes are relatively inexpensive.
Conclusions
We report polysulfone dialysis membrane-induced thrombocytopenia, which resolved with cellulose triacetate dialyzer substitution. The etiology of thrombocytopenia induced by polysulfone membrane is unclear, but substituting this membrane with a cellulose triacetate dialyzer is a reasonable first step in treating this disorder.
Tables
Table 1.. Laboratory values.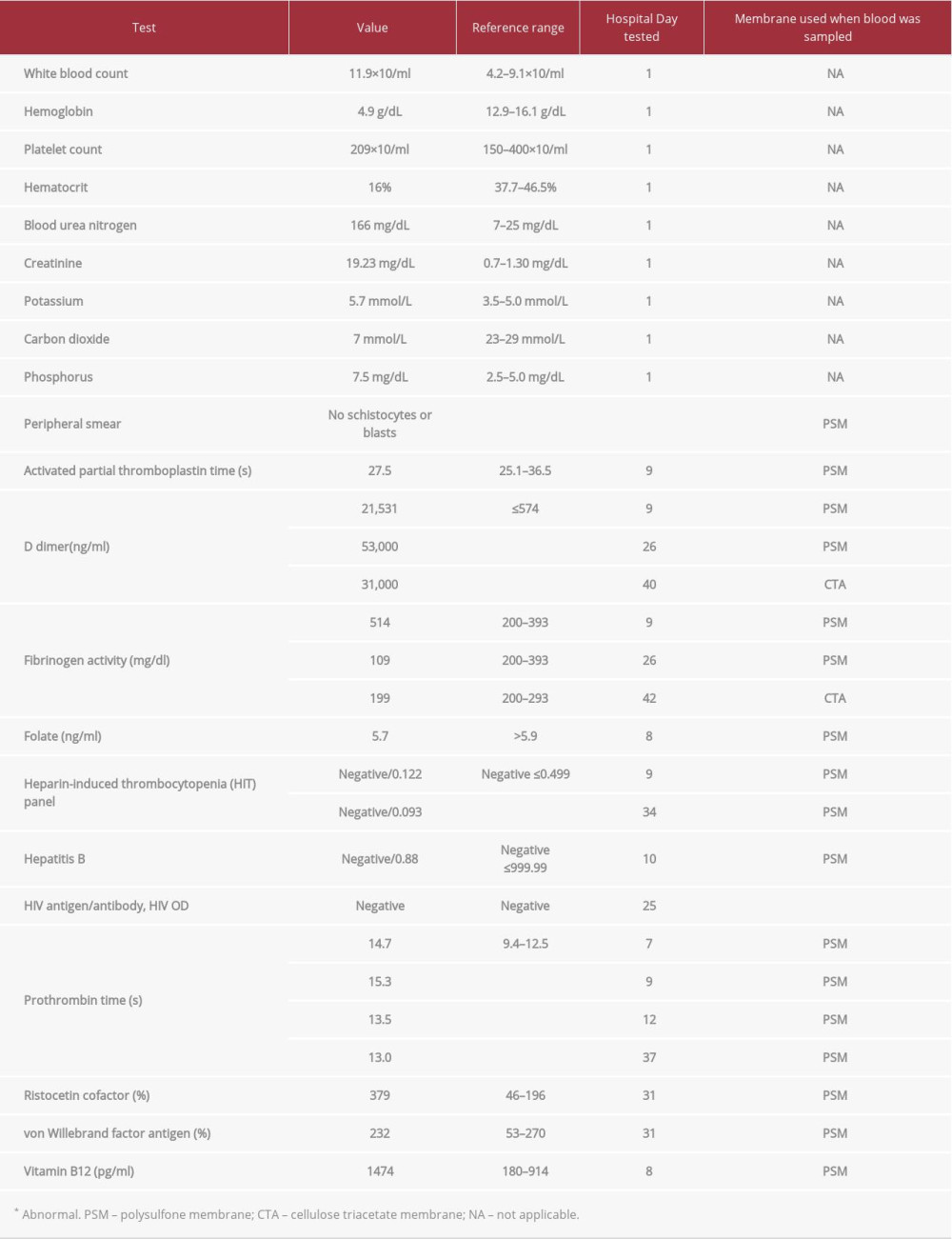
References:
1.. Ferreira JA, Johnson DW, The incidence of thrombocytopenia associated with continuous renal replacement therapy in critically ill patients: Ren Fail, 2015; 37(7); 1232-36
2.. Hoenich NA, Woffindin C, Mathews JN, Vienken J, Biocompatibility of membranes used in the treatment of renal failure: Biomaterials, 1995; 16(8); 587-92
3.. Posadas MA, Hahn D, Schleuter W, Paparello J, Thrombocytopenia associated with dialysis treatments: Hemodial Int, 2011; 15(3); 416-23
4.. Olafiranye F, Kyaw W, Olafiranye O, Resolution of dialyzer membrane-associated thrombocytopenia with use of cellulose triacetate membrane: A case report: Case Rep Med, 2011; 2011; 134295
5.. Daugirdas JT, Bernardo AA, Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia: Kidney Int, 2012; 82(2); 147-57
6.. Crowther MA, Cook DJ, Meade MO, Thrombocytopenia in medical-surgical critically ill patients: Prevalence, incidence, and risk factors: J Crit Care, 2005; 20(4); 348-53
7.. De Prada L, Lee J, Gillespie A, Benjamin J, Thrombocytopenia associated with one type of polysulfone hemodialysis membrane: A report of 5 cases: Am J Kidney Dis, 2013; 61(1); 131-33
8.. Liu S, Shi W, Liang X, Cellulose triacetate dialyzer reduces platelet loss during continuous veno-venous hemofiltration: Blood Purif, 2010; 29(4); 375-82
9.. Kiaii M, Djurdjev O, Farah M, Use of electron-beam sterilized hemodialysis membranes and risk of thrombocytopenia: JAMA, 2011; 306(15); 1679-87
10.. Filiopoulos V, Vlassopoulos D, Hemodialysis-associated thrombocytopenia: A multifactorial and idiosyncratic complication: Am J Kidney Dis, 2013; 61(5); 845
11.. Duayer IF, Araujo M, Nihei CH, Dialysis-related thrombocytopenia: A case report: J Bras Nefrol, 2021 [Online ahead of print]
12.. Post JB, Thrombocytopenia associated with use of a biocompatible hemo-dialysis membrane: A case report: Am J Kidney Dis, 2010; 55(6); e25-28
13.. Muir KB, Packer CD, Thrombocytopenia in the setting of hemodialysis using biocompatible membranes: Case Rep Med, 2012; 2012; 358024
14.. Kobari E, Terawaki H, Takahashi Y, Dialyzer-related thrombocytopenia due to a polysulfone membrane: Intern Med, 2016; 55(8); 965-68
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








