07 October 2021: Articles 
-chiro-inositol Induces Ovulation in Non-Polycystic Ovary Syndrome (PCOS), Non-Insulin-Resistant Young Women, Likely by Modulating Aromatase Expression: A Report of 2 Cases
Unknown etiology, Unusual clinical course, Unusual setting of medical care
Maria Salomé Bezerra Espinola123A*, Antonio Simone LaganàDOI: 10.12659/AJCR.932722
Am J Case Rep 2021; 22:e932722
Abstract
BACKGROUND: Anovulation consists in the lack of oocyte release during the menstrual cycle, leading to an irregular menstrual cycle. Untreated chronic anovulation is one of the major causes of female infertility and can induce hypoestrogenism. Different etiological factors can contribute to anovulation; therefore, the clinical approaches to manage this condition should take into account the specific patient characteristics. Oral ovulation-inducing agents are first-line treatments for most anovulatory patients. Drugs used include selective estrogen receptor modulators (SERMs) such as clomiphene citrate and aromatase inhibitors (AIs) such as letrozole. The latter, in particular, halts the estrogen biosynthesis by blocking the activity of steroidogenic enzyme aromatase, which catalyzes the conversion of androgens to estrogens. Similarly, d-chiro-inositol (DCI) modulates the activity of aromatase by reducing the corresponding gene expression, and DCI supplementation was successfully used to induce ovulation in anovulatory PCOS patients. Here, we report the use of DCI to induce ovulation in non-PCOS anovulatory oligomenorrheic women.
CASE REPORT: Two young non-PCOS anovulatory oligomenorrheic women received treatment with high-dose (1200 mg) DCI for 6 weeks. Based on an initial evaluation, both patients had normal hormone levels and were non-insulin-resistant. Ovulation assessment was based on the increment of progesterone and LH levels, as well as on the endometrial thickening. Also, the treatment with DCI resulted in a reduction of testosterone levels relative to baseline values.
CONCLUSIONS: After the 6-week treatment with 1200 mg DCI, ovulation was restored in both women, as confirmed by increased progesterone and LH and endometrial thickening.
Keywords: Anovulation, aromatase, Ovulation Induction, Female, Humans, Inositol, Ovulation
Background
Anovulation entails the lack of oocyte release during the menstrual cycle, with consequent oligomenorrhea or amenorrhea [1]. When untreated, chronic anovulation becomes a leading cause of female infertility, accounting for up to 30% of couples who fail to conceive, and often determines conditions related to a systemic lack of estrogens [2–4]. As anovulation comes with diverse etiological factors [1,5], the condition should be treated according to the specific patient characteristics. To facilitate the therapeutic approach, the World Health Organization (WHO) proposed a 3-group classification of infertile anovulatory women [6], based on their gonadotropin and estrogen levels [7]. WHO group II represents 80–90% of cases, including patients with polycystic ovary syndrome (PCOS) and non-PCOS patients with dysfunctional hypothalamic pituitary ovarian axis [8]. The latter, in particular, have physiologic basal levels of hormones but inconsistent release of gonadotropin-releasing hormone (GnRH) during ovarian folliculogenesis [2]. Low GnRH levels result in insufficient release of FSH and LH to stimulate follicular growth and estradiol biosynthesis in the granulosa. As a consequence, endometrial lining fails to proliferate, and menstrual bleeding often does not occur.
Oral ovulation-inducing agents are first-line treatments for WHO group II anovulatory patients [9,10]. They primarily include selective estrogen receptor modulators (SERMs), such as clomiphene citrate (CC), which prevent the interaction of estrogens with their receptors [11]. Aromatase inhibitors (AIs) [12], such as letrozole, are also widely used to induce ovulation, as they block estrogen biosynthesis by inhibiting the activity of the enzyme CYP19A1, commonly known as aromatase. Albeit with different mechanisms, the final effect of these pharmacological approaches is to reduce the negative feedback of estrogens on the hypothalamus, thereby stimulating the release of GnRH and gonadotropins.
Two isomers from the inositol family, myo-inositol (MI) and d-chiro-inositol (DCI), have a crucial role in ovarian physiology [13]. Studies on patients undergoing assisted reproduction procedures found that both compounds are necessary [14–16], and that a specific ratio is required for optimal oocyte development [17,18]. As phosphorylated derivatives, MI and DCI are second messengers of insulin [19], but also have specific individual roles: while MI was extensively studied as a second messenger of FSH, insulin, and TSH [20], the understanding of the involvement of DCI in intracellular physiological processes is more recent [21]. In particular, DCI modulates the activity of aromatase and exerts some of the effects normally associated with AIs [22]. Indeed, due to this effect on aromatase, DCI may increase the androgen pool at the expense of estrogens in the ovaries. As suggested by Carlomagno et al [23,24] the ovaries never display insulin resistance. As a consequence, in insulin-resistant PCOS patients, those tissues show an enhanced epimerase activity and an increased DCI content (the “ovarian paradox”). Therefore, DCI supplementation for prolonged times (>3 months) and in high doses (>1200 mg) can exacerbate the hyperandrogenism that is a feature of PCOS women. Thus, evaluating the clinical status of the patient before starting DCI supplementation should be mandatory.
However, since short-term treatments with DCI successfully induce ovulation in women with PCOS [25], our interest is to determine whether it could be used in non-PCOS, non-IR WHO group II anovulatory patients with the same purpose. Here, we report 2 cases of young oligomenorrheic women with such characteristics, who achieved ovulation after a 6-week treatment with a high dose (1200 mg) of d-chiro-inositol. We hypothesized that a hypothalamic feedback is possibly involved, mediated by the supposedly negative effect of DCI on aroma-tase enzyme, in a way similar to letrozole.
The present investigation was approved by the institutional review board of Alma Res IVF Center (approval #002/2020), and written informed consent was signed by both participants.
Case Reports
CASE 1:
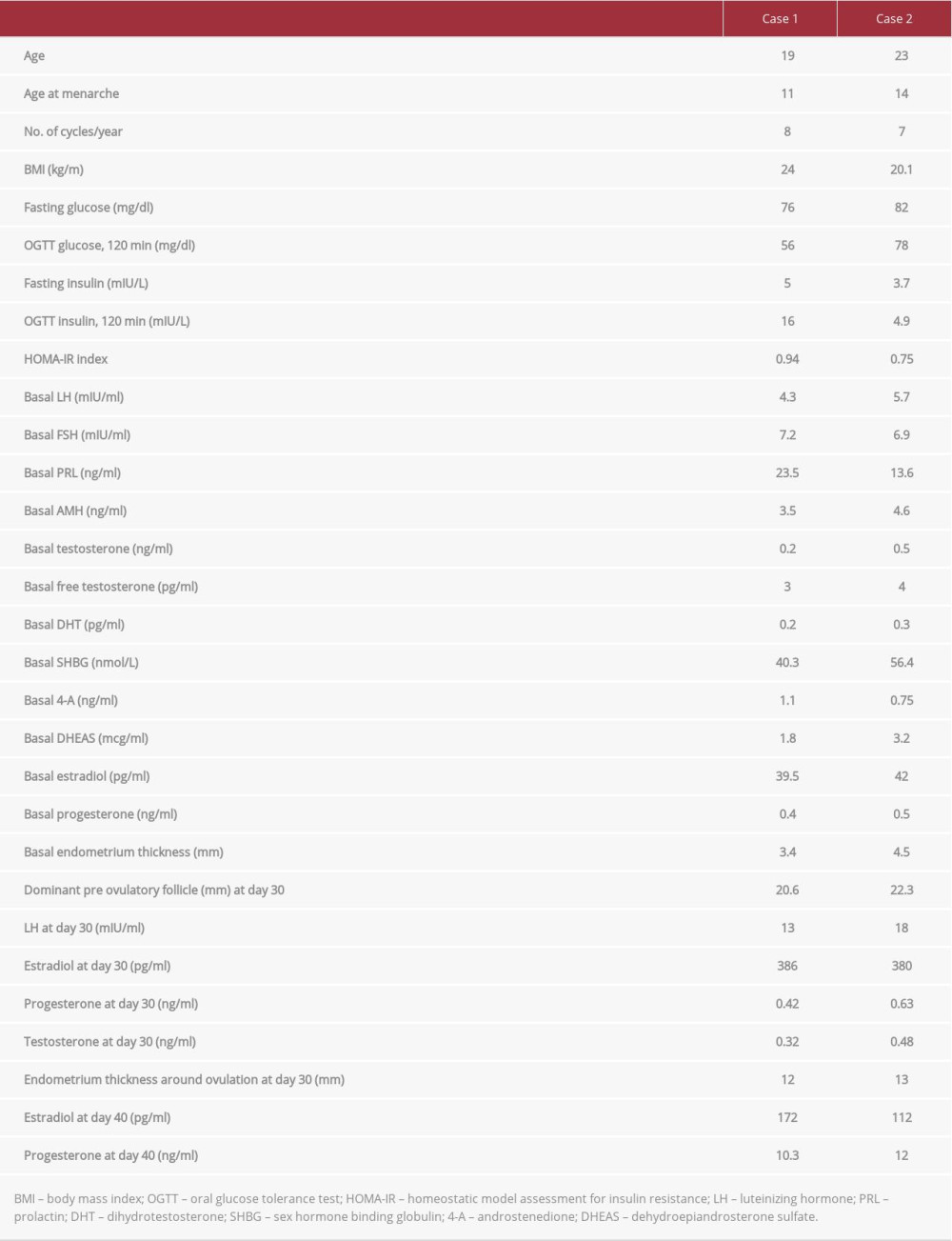
A 19-year-old woman with a diagnosis of anovulatory oligomenorrhea was referred to our center. The patient was euthyroid and had hormone levels in the physiologic range (Table 1). Transvaginal ultrasound (US) revealed multifollicular ovaries with the absence of cysts. On this basis, anovulation was associated with altered hypothalamic–pituitary–ovarian response (anovulatory WHO group II). Oral glucose tolerance test (OGTT) revealed physiologic blood glucose clearance and normal insulin levels, and the associated HOMA-IR index denotes the absence of insulin resistance. Moreover, basal estradiol levels were in the early follicular phase range. The patient was treated for 3 months with 400 mcg q.d. oral folic acid (Folidex, Italfarmaco, Milan, Italy), without evidence of ovulation (based on serum progesterone measured at 10 days interval <1.0 ng/ ml). The treatment was then modified with a galenical combination of 400 mcg folic acid and 1200 mg d-chiro-inositol (once daily) and further extended for 6 weeks, starting at the follicular phase of the cycle (progesterone < 1.0 ng/ml). Monitoring was carried out every 10 days by vaginal ultrasound and serum progesterone, estradiol, LH, and testosterone assessment. Ovulation was confirmed by increased LH and progesterone levels at days 30 and 40, respectively, accompanied by testosterone decrease and endometrial thickening. The patient menstruated 52 days after starting treatment. testosterone decrease and endometrial thickening. The patient menstruated 50 days after starting treatment.
CASE 2:
A 23-year-old woman with a diagnosis of anovulatory oligomenorrhea was referred to our center. The patient was euthyroid and had hormone levels in the physiologic range (Table 1). US revealed multifollicular ovaries with the absence of cysts. On this basis, anovulation was associated with altered hypothalamic–pituitary–ovarian response (anovulatory WHO group II). OGTT revealed physiologic blood glucose clearance and normal insulin levels, and the associated HOMA-IR index denotes the absence of insulin resistance. Moreover, basal estradiol levels were in the early follicular phase range. The patient was treated for 3 months with 400 mcg q.d. oral folic acid (Folidex, Italfarmaco, Milan, Italy), without evidence of ovulation (based on serum progesterone measured at 10-day intervals <1.0 ng/ml). The treatment was then modified with a galenical combination of 400 mcg folic acid and 1200 mg d-chiro-inositol (once daily) and further extended for 6 weeks, starting at the follicular phase of the cycle (progesterone <1.0 ng/ml). Monitoring was carried out every 10 days by vaginal ultrasound and serum progesterone and estradiol assessment. Ovulation was confirmed by increased LH and progesterone levels at days 30 and 40, respectively, accompanied by
Discussion
We describe 2 cases of successful utilization of DCI to induce ovulation in women that were non-PCOS and non-IR. The ovula-tory cycle occurred after about 6 weeks of treatment, accompanied by increased progesterone levels and thickened endome-trial lining. To further support the induction of ovulation, also LH levels increased while testosterone importantly decreased.
The positive effect of DCI on ovulation was first described by Nestler and co-workers in obese, insulin-resistant PCOS patients with anovulation [25]. Administration of 1200 mg/day of DCI for 6–8 weeks resulted in a significantly higher number of patients that ovulated compared to placebo. Moreover, DCI treatment led to a significant reduction of insulin and systemic androgen levels. Interestingly, the same authors later proved that the effect of DCI is dose-dependent, and that amounts below 1200 mg/day were less effective in inducing ovulation [26]. The results of the present case report mirrored the findings recorded by Nestler and co-workers. Accordingly, dose and timing of DCI treatment reflect the protocol of DCI supplementation applied in Nestler’s study.
Decreased insulin levels accounted for such results, as DCI-containing phosphoglycans are intracellular insulin mediators and increase cell sensitivity [27–31]. Indeed, insulin sensitizers such as metformin proved effective in inducing ovulation, especially in women with hyperinsulinemia [32,33]. Metformin was also successfully used to potentiate the effect of other ovulation-inducing agents in stimulation protocols [34,35]. Insulin directly activates aromatase, and elevated insulin levels increase the biosynthesis of estrogens [36]. Unsurprisingly, PCOS women often present with a hyper-estrogenic state [36]. The decrease of systemic levels of estrogens results in less negative feedback on the hypothalamus, with consequent release of GnRH [12].
Independent of the insulin status, current approaches to induce ovulation rely either on exogenous gonadotropin injection or on the anti-estrogenic activity necessary to stimulate the hypothalamic–pituitary response [34,37]. Clomiphene citrate is still the most widely used drug to stimulate ovulation [38]. It is a SERM and binds to the estrogen receptors in the hypothalamus, preventing the negative feedback of estrogens and allowing the release of FSH, which stimulates the follicles to grow. A major drawback of CC use is the lack of specificity, as other estrogen-dependent organs and tissue are affected. In particular, CC inhibits endometrial thickening and cervical mucus formation [39,40] at the expense of fertility [41]. In fact, a poor-quality endometrium for embryo implantation may undermine the advantages of restoring the ovulatory function in assisted reproductive technology (ART) protocols. Letrozole is a third-generation AI that prevents estrogen biosynthesis by blocking the activity of aromatase, the enzyme responsible for conversion of androgens [42,43]. In addition to enhancing the hypothalamic response, it increases the FSH sensitivity of the ovaries [44]. Due to its shorter half life, compared to CC, letrozole does not interfere with endometrial proliferation and reduces the occurrence of multiple pregnancies in ART protocols [45,46]. However, such use of letrozole is off label in many countries and concerns have been raised regarding possible negative fetal outcomes connected with its use, although such concerns are not supported by experimental evidence [47].
In addition to mediating the intracellular signaling of insulin, DCI modulates the ratio of androgen-to-estrogen biosynthesis by reducing the activity of aromatase [48]. Although long known, Sacchi and co-workers only recently proved this effect, demonstrating in vitro that DCI downregulates the gene expression of the enzyme [49].
Considering that our 2 patients were normo-insulinemic, it is unlikely that insulin regulation had a role in restoring the ovulatory function. We rather believe that the observed effect of DCI treatment on aromatase activity yields a lower biosynthesis of estrogens and stimulates the release of GnRH. On these premises, we also suggest that DCI treatment may be evaluated to induce ovulation in non-PCOS, non-IR women, as concluded by Gambioli and colleagues, who reported on different doses and times for DCI supplementation [50].
Conclusions
As long-term administration of high doses of DCI are proved to increase testosterone levels, DCI supplementation to PCOS women should be carefully evaluated, and should be limited to short-term treatments for all other types of patients. Indeed, non-IR and non-PCOS women may benefit from adequate DCI treatment. Here, we provide evidence of DCI’s efficacy in inducing ovulation in such patients. Our findings suggest that DCI is a safe alternative approach to pharmaceuticals to stimulate ovulatory function in anovulatory women. Further controlled studies on an appropriate number of patients are necessary to confirm our preliminary observations.
References:
1.. Hamilton-Fairley D, Taylor A, Anovulation: BMJ, 2003; 327; 546-49
2.. Meczekalski B, Katulski K, Czyzyk A, Functional hypothalamic amenorrhea and its influence on women’s health: J Endocrinol Invest, 2014; 37; 1049-56
3.. Shufelt CL, Torbati T, Dutra E, Hypothalamic amenorrhea and the long-term health consequences: Semin Reprod Med, 2017; 35; 256-62
4.. Baker L, Meldrum KK, Wang M, The role of estrogen in cardiovascular disease: J Surg Res, 2003; 115; 325-44
5.. Chandeying P, Pantasri T, Prevalence of conditions causing chronic anovulation and the proposed algorithm for anovulation evaluation: J Obstet Gynaecol Res, 2015; 41; 1074-79
6.. : Advances in methods of fer-tility regulation: report of a WHO Scientific Group [meeting held in Geneva from 11 to 15 December 1972], 1973, Geneva, World Health Organization
7.. Insler V, Melmed H, Mashiah S, Functional classification of patients selected for gonadotropic therapy: Obstet Gynecol, 1968; 32; 620-26
8.. Gorthi S, Balen AH, Tang T, Current issues in ovulation induction: The Obstetrician & Gynaecologist, 2012; 14; 188-96
9.. Shapiro A, Kashani B, Seungdamrong A, Oral ovulation induction agents: Topics in Obstetrics & Gynecology; 2017; 37
10.. Tulandi T, Weibel HS, Ovulation induction: Clinical reproductive medicine and surgery: A practical guide, 2017; 289-98, Cham, Springer International Publishing
11.. Tanbo T, Mellembakken J, Bjercke S, Ovulation induction in polycystic ovary syndrome: Acta Obstet Gynecol Scand, 2018; 97; 1162-67
12.. Casper RF, Mitwally MF, Review: Aromatase inhibitors for ovulation induction: J Clin Endocrinol Metab, 2006; 91; 760-71
13.. Unfer V, Orru B, Monastra G, Inositols: From physiology to rational therapy in gynecological clinical practice: Expert Opin Drug Metab Toxicol, 2016; 12; 1129-31
14.. Facchinetti F, Espinola MSB, Dewailly D, Breakthroughs in the use of inositols for assisted reproductive treatment (ART): Trends Endocrinol Metab, 2020; 31; 570-79
15.. Nordio M, Proietti E, The combined therapy with myo-inositol and d-chiroinositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inositol supplementation alone: Eur Rev Med Pharmacol Sci, 2012; 16; 575-81
16.. Colazingari S, Treglia M, Najjar R: Arch Gynecol Obstet, 2013; 288; 1405-11
17.. Minozzi M, Nordio M, Pajalich R: Eur Rev Med Pharmacol Sci, 2013; 17; 537-40
18.. Nordio M, Basciani S, Camajani E: Eur Rev Med Pharmacol Sci, 2019; 23; 5512-21
19.. Asplin I, Galasko G, Larner J, chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects: Proc Natl Acad Sci USA, 1993; 90; 5924-28
20.. Bizzarri M, Fuso A, Dinicola S, Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease: Expert Opin Drug Metab Toxicol, 2016; 12; 1181-96
21.. Laganà AS, Garzon S, Unfer V: Expert Opin Drug Metab Toxicol, 2020; 16; 703-10
22.. Laganà AS, Unfer V: Eur Rev Med Pharmacol Sci, 2019; 23; 10575-76
23.. Carlomagno G, Unfer V, Roseff S: Fertil Steril, 2011; 95; 2515-16
24.. Unfer V, Dinicola S, Laganà AS, Altered ovarian inositol ratios may account for pathological steroidogenesis in PCOS: Int J Mol Sci, 2020; 21; 7157
25.. Nestler JE, Jakubowicz DJ, Reamer P: N Engl J Med, 1999; 340; 1314-20
26.. Nestler JE, Gunn R, Bates S: Fertil Steril, 2001; 76; S110-11
27.. Nestler JE, Jakubowicz DJ, Reamer P: N Engl J Med, 1999; 340; 1314-20
28.. Laganà AS, Barbaro L, Pizzo A: Arch Gynecol Obstet, 2015; 291; 1181-86
29.. Gupta A, Jakubowicz D, Nestler JE: Metab Syndr Relat Disord, 2016; 14; 391-96
30.. Fonteles MC, Huang LC, Larner J: Diabetologia, 1996; 39; 731-34
31.. Larner J: Int J Exp Diabetes Res, 2002; 3; 47-60
32.. De Leo V, la Marca A, Ditto A, Effects of metformin on gonadotropin-induced ovulation in women with polycystic ovary syndrome: Fertil Steril, 1999; 72; 282-85
33.. , Role of metformin for ovulation induction in infertile patients with poly-cystic ovary syndrome (PCOS): A guideline: Fertil Steril, 2017; 108; 426-41
34.. Wang R, Kim BV, van Wely M, Treatment strategies for women with WHO group II anovulation: Systematic review and network meta-analysis: BMJ, 2017; 356; j138
35.. Agrawal A, Mahey R, Kachhawa G, Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: Randomized controlled trial: Gynecol Endocrinol, 2019; 35; 511-14
36.. la Marca A, Morgante G, Palumbo M, Insulin-lowering treatment reduces aromatase activity in response to follicle-stimulating hormone in women with polycystic ovary syndrome: Fertil Steril, 2002; 78; 1234-39
37.. Palomba S, Orio F, Zullo F, Ovulation induction in women with polycystic ovary syndrome: Fertil Steril, 2006; 86; S26-27
38.. Lindheim SR, Glenn TL, Smith MC, Ovulation induction for the general gynecologist: J Obstet Gynaecol India, 2018; 68; 242-52
39.. Nakamura Y, Ono M, Yoshida Y, Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium: Fertil Steril, 1997; 67; 256-60
40.. Randall JM, Templeton A, Cervical mucus score and in vitro sperm mucus interaction in spontaneous and clomiphene citrate cycles: Fertil Steril, 1991; 56; 465-68
41.. Dickey RP, Olar TT, Taylor SN, Relationship of endometrial thickness and pattern to fecundity in ovulation induction cycles: Effect of clomiphene citrate alone and with human menopausal gonadotropin: Fertil Steril, 1993; 59; 756-60
42.. Mitwally MFM, Casper RF, Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate: Fertil Steril, 2001; 75; 305-9
43.. Pritts EA, Letrozole for ovulation induction and controlled ovarian hyper-stimulation: Curr Opin Obstet Gynecol, 2010; 22; 289-94
44.. Bayar Ü, Tanrıverdi HA, Barut A, Letrozole vs. clomiphene citrate in patients with ovulatory infertility: Fertil Steril, 2006; 85; 1045-48
45.. Legro RS, Brzyski RG, Diamond MP, Letrozole versus clomiphene for infertility in the polycystic ovary syndrome: N Engl J Med, 2014; 371; 119-29
46.. Thomas S, Woo I, Ho J, Ovulation rates in a stair-step protocol with Letrozole vs clomiphene citrate in patients with polycystic ovarian syndrome: Contracept Reprod Med, 2019; 4; 20
47.. Franik S, Eltrop SM, Kremer JAM, Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome: Cochrane Database Syst Rev, 2018; 5; CD010287
48.. Nestler JE, Regulation of the aromatase activity of human placental cytotrophoblasts by insulin, insulin-like growth factor-I, and -II: J Steroid Biochem Mol Biol, 1993; 44; 449-57
49.. Sacchi S, Marinaro F, Tondelli D: Reprod Biol Endocrinol, 2016; 14; 52
50.. Gambioli R, Forte G, Aragona C: Eur Rev Med Pharmacol Sci, 2021; 25; 438-46
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250