16 October 2021: Articles 
Difficulties in Diagnosing and Treating Disseminated Bacillus Calmette-Guérin (BCG) Infection After Intravesical BCG Therapy in a Patient with Liver Cirrhosis: A Case Report
Challenging differential diagnosis, Adverse events of drug therapy, Rare coexistence of disease or pathology
Christos Vallilas1BCDE, Maria Zachou2BCDE, Philippos Dolkiras3BDE, Stratigoula Sakellariou4BDE, Costas A. Constantinou5BCDE, Pagona Flevari6DE, Amalia Anastasopoulou7CDEF, Theodoros Androutsakos8ABCDEF*DOI: 10.12659/AJCR.933006
Am J Case Rep 2021; 22:e933006
Abstract
BACKGROUND: Bladder cancer (BC) is the second most common cancer involving the urinary system. In non-muscle-invading BC, transurethral resection of a bladder tumor followed by intravesical immunotherapy with Bacillus Calmette-Guérin (BCG) is the usual treatment. Disseminated (or systemic) BCG infection (BCGitis) represents the most severe adverse effect of intravesical BCG therapy, presenting with high-grade fever, with or without symptoms in the urinary tract, leading to severe sepsis and death if left untreated. The treatment of choice consists of isoniazid, rifampicin, and ethambutol (with or without corticosteroids) for 6 months, and the recovery rate is extremely high. Given the fact that these drugs are hepatotoxic, treating a patient with liver cirrhosis is challenging.
CASE REPORT: We present a patient with a medical history of BC treated with transurethral resection and intravesical BCG therapy, presenting with fever, transaminasemia, and generalized weakness. Liver and bone marrow biopsies revealed liver cirrhosis and granulomas in both organs. A diagnose of BCGitis was made and the patient was treated with isoniazid, rifampicin, and ethambutol; rifampicin was substituted with moxifloxacin after 1 month due to worsening of liver laboratory results, and moxifloxacin was substituted with levofloxacin later on due to tonic-clonic seizures. The patient was treated for 4 more months with levofloxacin and for 7 more months with isoniazid and ethambutol, with no other adverse effects, preserving liver function and achieving cure of BCGitis.
CONCLUSIONS: We present the case of a cirrhotic patient presenting with fever and deterioration of liver laboratory results, found to have BCGitis, and discuss possible difficulties in diagnosing and treating such patients.
Keywords: BCG and Salmonella Infection, Disseminated, Isoniazid, Liver Cirrhosis, Rifampin
Background
Bladder cancer (BC) is the ninth most common malignancy worldwide and the second most common one involving the urinary system; however, in middle-aged and elderly men, BC is the second most common malignancy, after only prostate cancer [1–4]. The majority (75–80%) of BCs are non-muscle-invasive (NMIBC) and the treatment strategy includes transurethral resection of bladder tumor (TURBT), usually followed by intravesical immunotherapy with Bacillus Calmette-Guérin (BCG), which is an attenuated strain of
Despite the efficacy of intravesical BCG therapy, most patients report local, mild, early complications after instillation, like cystitis (81%), macroscopic hematuria (15%), and urination frequency [9–13]. Although BCG therapy is usually well-tolerated, local and systemic complications can occur [9,10,13]. Local complications involve the genitourinary tract in the form of epididymitis and granulomatous prostatitis, while systemic ones include short-term fever in 5–30% of patients, as well as allergic reactions, reactive arthritis, drug-induced liver injury, granulomatous hepatitis, and nephritis, all with a prevalence of less than 1% [10,12–15].
Disseminated (or systemic) BCG infection (BCGitis) is the most severe adverse effect of intravesical BCG therapy. BCGitis has an incidence of 3% to 7% and presents with high-grade fever and/or various organ involvement with or without symptoms from the urinary tract, leading to severe sepsis and death if left untreated [9,10,16–19]. BCGitis should be suspected in any patient who develops moderate-to-severe genitourinary or systemic symptoms following ≥1 instillation of intravesical BCG when no alternative diagnosis can be established. Microbiological documentation of the infection is required for definitive diagnosis; however, it has low sensitivity, and when histopathology is available, granulomas are commonly found [17]. Due to the aforementioned difficulties, empirical treatment is commonly started upon suspicion; in this setting, improvement of symptoms after treatment initiation is mandatory for establishing the diagnosis [9,14,17]. The treatment of choice for BCGitis consists of isoniazid, rifampicin, and ethambutol (alone or in combination with corticosteroids) for 6 months, and the recovery rate is extremely high [17,20–24]. Unfortunately, all of the above-mentioned drugs are hepatotoxic and can cause liver dysfunction, a problem most often encountered in cirrhotic patients [25]. Here, we report the first case of a cirrhotic patient diagnosed with BCGitis with liver and bone marrow involvement, and we discuss potential therapeutic challenges related to the co-existence of BCGitis and liver cirrhosis.
Case Report
A 62-year-old non-smoker, male patient was admitted to Laiko General Hospital in Athens, Greece, due to a 6-month history of fever and generalized weakness. He had a medical history of non-muscle-invading BC, diagnosed 2 years before presentation and treated with transurethral resection (TURBT) followed by 6 weeks of intravesical therapy with BCG. His medical anamnesis was also notable for coronary artery disease and dyslipidemia. He reported consuming moderate amounts of alcohol daily for the past 20 years, stopping about 4 months ago.
Approximately 2 months before presentation, the patient was hospitalized in another hospital due to fever up to 38.5°C, anemia, and generalized weakness. During his stay there, a cystoscopy was performed that revealed BC recurrence and he received a second course of 6 weekly intravesical BCG infusions. His fever was attributed to BC recurrence, so no further diagnostic evaluation was performed at that time. Due to fever persistence and generalized weakness after treatment completion, he was admitted to our hospital for further evaluation and management.
Upon admission, the patient had an axillary temperature of 37.5°C, a blood pressure of 110/80 mmHg, a heart rate of 93 beats per minute, an oxygen saturation of 89% on ambient air, and a respiratory rate of 22 breaths per minute. On physical examination, he was mildly lethargic with no focal neurological deficits or signs. Lung auscultation revealed bilateral crackles and abdomen examination revealed hepato-splenomegaly with no pain during palpation. The rest of his clinical examination was unremarkable.
Routine laboratory blood results showed mild pancytopenia, elevated serum gamma glutamyl-transferase (GGT) and alkaline phosphatase (ALP) levels, as well as mildly elevated serum C-reactive protein (CRP) aspartate (AST) and normal alanine (ALT) aminotransferases levels (Table 1). Urinary testing revealed pyuria and mild hematuria, whereas the urine culture was sterile.
A chest X-ray was performed that demonstrated bilateral pleural fluid accumulation, while abdominal ultrasound revealed a cirrhotic liver, splenomegaly, and mild ascites. No focal hepatic lesions were found. Computed tomography (C/T) scans of the chest and abdomen confirmed the aforementioned findings, without revealing portal or hepatic veins thrombosis.
Ascitic fluid analysis was consistent with portal hypertension-induced ascites (serum-to-ascites albumin gradient of 2.1 g/dl), with no signs of infection or malignancy. Testing for hepatitis B and hepatitis C infections, Leishmaniasis, Q fever, and Brucellosis were negative, as were anti-nuclear, anti-smooth muscle, and anti-mitochondrial antibodies in serum. Serum iron studies, ferritin, ceruloplasmin level, and 24-h urine copper excretion were within normal limits. Quantiferon testing was negative and an echocardiogram was also normal.
Due to pancytopenia, a bone marrow biopsy was performed. Additionally, a liver biopsy was performed due to progressive deterioration of liver biochemistry results. The bone marrow biopsy revealed non-caseating granulomas and Langhans giant cells (Figure 1A, 1B), whereas the liver biopsy showed bridging fibrosis along with multiple, small, non-caseating granulomas and mild portal and lobular inflammation (Figure 2A, 2B).
Given the recent history of intravesical BCG therapy and the histological findings of liver and bone marrow biopsies, a diagnosis of BCGitis was made. The patient was started on isoniazid, rifampicin, and ethambutol, had a rapid resolution of symptoms, and was discharged from the hospital 4 days after treatment initiation.
One month after discharge, the patient had further deterioration of GGT and ALP serum levels (Table 1), so rifampicin was discontinued and moxifloxacin was initiated instead. Four weeks after treatment modification, the patient experienced an episode of loss of consciousness with gaze fixation that resolved spontaneously a few minutes later. A brain magnetic resonance imaging (MRI) was ordered, which revealed no pathology, and moxifloxacin was substituted for levofloxacin.
During his next visits, the patient had a slow improvement of liver biochemistry and overall status, so levofloxacin was continued for 4 additional months and isoniazid and ethambutol was continued for 7 additional months (9 months in total). Three months after treatment completion, the patient was afebrile, in good overall status, with normal levels of serum AST, ALT, and ALP, and only mildly elevated GGT levels, with no progression of liver cirrhosis status as defined by MELD and CTP scores.
Discussion
We present the case of a patient with a history of BC treated with intravesical BCG who was concurrently diagnosed with disseminated BCGitis (with bone marrow and liver involvement).
BCGitis is a well-known adverse effect of intravesical BCG infusions. Incorrect instillation of BCG with iatrogenic trauma or concurrent urinary infection, previous urinary interventions, and the presence of breaches in the bladder are the most common risk factors [11,16,17,26–29]. Since the attenuated
BCGitis should be suspected in every patient having received intravesical BCG infusions, presenting with fever and/or organ involvement, with or without symptoms in the genitourinary tract. However, establishing the diagnosis can be quite challenging, needing, apart from the history of BCG exposure, exclusion of all other causes of fever, as well as clinical improvement after treatment initiation, since microbiological and/or histopathological documentation is rarely feasible. Nevertheless, in our patient, the clinical suspicion was supported by liver and bone marrow histology, which found noncaseating granulomas. Interestingly, unlike tuberculosis, infection with
Initial treatment for BCGitis consists of isoniazid, rifampicin, and ethambutol, alone or in combination with corticosteroids, for 6 months, and the infection usually resolves with no sequelae. Corticosteroid use is controversial and is usually reserved for severe cases of miliary involvement and respiratory failure. Treating a cirrhotic patient poses many challenges, since anti-tuberculosis treatment is accompanied with significant hepatotoxicity. Isoniazid is metabolized in the liver, leading to hepatotoxic metabolites in the form of acetyl hydrazine and hydrazine, which cause hepatocellular steatosis and necrosis [25,41,42], with an incidence of 0.6% when used as mono-therapy [25,43], although severe isoniazid-induced hepatotoxicity seems to be heavily under-reported [44]. Rifampicin usually causes transient transaminitis that is thought to be benign; however, it has also been associated with centrilobular parenchymal necrosis and elevation of conjugated bilirubin due to cholestasis [45,46], leading to a 4-fold higher incidence of hepatotoxicity when used in combination with isoniazid [47]. Interestingly, according to Park et al, this incidence increases to 17% when a liver disease coexists, irrespective of the presence or absence of cirrhosis [48]. In contrast, ethambutol is not associated with clinically important hepatotoxic effects [25,49].
Due to these adverse effects, treating a patient with liver cirrhosis for BCGitis involves the risk of decompensation or even acute-on-chronic liver failure [25]. According to WHO guidelines, the number of hepatotoxic agents that can be used in a cirrhotic patient depends on the severity of liver disease [50]. Regarding anti-tuberculosis (TB) drugs, some authors propose the use of isoniazid and rifampicin for patients with Child-Pugh ≤7, isoniazid or rifampicin for Child-Pugh 8–10, and none of the hepatotoxic anti-TB drugs for patients with Child-Pugh ≥11 [51,52]. Since hepatotoxicity usually occurs during the first 2 months of treatment, close monitoring of transaminases’ levels is mandatory in that period [42,51,52].
Due to the aforementioned problems, our patient was started on isoniazid, rifampicin, and ethambutol, according to guidelines. The patient had a deterioration of liver enzymes serum levels during his first follow-up visit, so rifampicin was changed to moxifloxacin.
With regards to fluoroquinolones (FQs), which are the most commonly used second-line agents, a large case-control safety study showed no significant association between moxifloxacin and levofloxacin exposure and hepatotoxicity risk, while Ho et al found that introduction of FQ in patients with hepatitis induced by first-line agents did not cause additional hepatotoxicity [53,54]. On the contrary, Paterson et al found increased risk of acute liver injury among older patients [55]. Given these results, FQs may be a reasonable option in patients with hepatitis after exposure to first-line anti-TB agents.
Even though this regimen was considered to be safe for a cirrhotic patient, our patient developed seizures attributed to moxifloxacin treatment, which resolved after discontinuation of the medication. Although levofloxacin, ofloxacin, and moxifloxacin have the lowest potential of inducing central nervous system adverse events among the FQ available, case reports exist attributing episodes of seizures to moxifloxacin treatment [56–59]. Our patient’s regimen was once more changed and levofloxacin was initiated instead of moxifloxacin, without other significant toxicities.
Although the usual treatment duration for BCGitis is 6 months, no treatment recommendations exist regarding immunocom-promised patients, since most data come from children with primary immunodeficiency syndromes for which regimens with various durations were used [60–62]. In a case report presented by Winger et al [63], a 16-year-old patient with systemic lupus erythematosus, diagnosed with intestinal
During his last visit, 3 months after treatment completion (12 months after treatment initiation), the patient was stable, with no fever or deterioration of cirrhosis status.
Conclusions
We report a unique case of concurrent diagnosis of BCGitis and liver cirrhosis in a patient with previous intravesical BCG administration for BC and discuss the difficulties in diagnosing and treating such a patient for BCGitis.
Figures
References:
1.. Antoni S, Ferlay J, Soerjomataram I, Bladder cancer incidence and mortality: A global overview and recent trends: Eur Urol, 2017; 71(1); 96-108
2.. Jochems SHJ, Reulen RC, van Osch FHM, Fruit consumption and the risk of bladder cancer: A pooled analysis by the Bladder Cancer Epidemiology and Nutritional Determinants Study: Int J Cancer, 2020; 147(8); 2091-100
3.. Ferlay J, Soerjomataram I, Dikshit R, Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Int J Cancer, 2015; 136(5); E359-86
4.. Jemal A, Bray F, Center MM, Global cancer statistics [published correction appears in Cancer J Clin. 2011;61(2):134134]: CA Cancer J Clin, 2011; 61(2); 69-90
5.. , Bladder cancer: Diagnosis and management of bladder cancer: © NICE (2015) Bladder cancer: Diagnosis and management of bladder cancer: BJU Int, 2017; 120(6); 755-65
6.. Fujita N, Hatakeyama S, Momota M, Safety and efficacy of efficacy of intensive instillation of low-dose pirarubicin vs. bacillus Calmette-Guérin in patients with high-risk non-muscle-invasive bladder cancer: Urol Oncol, 2020; 38(8); 684.e17-e24
7.. Pirzada MT, Ghauri R, Ahmed MJ, Outcomes of BCG induction in high-risk Non-Muscle-Invasive Bladder Cancer Patients (NMIBC): A retrospective cohort study: Cureus, 2017; 9(1); e957
8.. Balan D, Martha O, Chibelean CB, Comparison of 10-year overall survival between patients with G1 and G2 grade Ta bladder tumors: Medicine (Baltimore), 2018; 97(16); e0522
9.. Marques M, Vazquez D, Sousa S, Disseminated Bacillus Calmette-Guérin (BCG) infection with pulmonary and renal involvement: A rare complication of BCG immunotherapy. A case report and narrative review: Pulmonology, 2020; 26(6); 346-52
10.. Resel Folkersma LE, Belón Lopéz Tomasetti JA, Isorna Martínez de la Riva S, Caminero Luna JA, [Complications of endovesical treatment with BCG in a series of 200 patients.]: Arch Esp Urol, 1999; 52(9); 957-65 [in Spanish]
11.. Lamm DL, van der Meijden PM, Morales A, Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer: J Urol, 1992; 147(3); 596-600
12.. Parker SG, Kommu SS, Post-intravesical BCG epididymo-orchitis: Case report and a review of the literature: Int J Surg Case Rep, 2013; 4(9); 768-70
13.. Rischmann P, Desgrandchamps F, Malavaud B, Chopin DK, BCG intravesical instillations: Recommendations for side-effects management: Eur Urol, 2000; 37(Suppl. 1); 33-36
14.. Liu Y, Lu J, Huang Y, Ma L, Clinical spectrum of complications induced by intravesical immunotherapy of bacillus Calmette-Guérin for bladder cancer: J Oncol, 2019; 2019; 6230409
15.. Mathes J, Todenhöfer T, Managing toxicity of intravesical therapy: Eur Urol Focus, 2018; 4(4); 464-67
16.. Steg A, Leleu C, Debré B, Systemic bacillus Calmette-Guérin infection, ‘BCGitis’, in patients treated by intravesical bacillus Calmette-Guérin therapy for bladder cancer: Eur Urol, 1989; 16(3); 161-64
17.. Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: Incidence, risk factors, and outcome in a single-institution series and review of the literature: Medicine (Baltimore), 2014; 93(17); 236-54
18.. Gonzalez OY, Musher DM, Brar I, Spectrum of bacille Calmette-Guérin (BCG) infection after intravesical BCG immunotherapy: Clin Infect Dis, 2003; 36(2); 140-48
19.. Rawls WH, Lamm DL, Lowe BA, Fatal sepsis following intravesical bacillus Calmette-Guerin administration for bladder cancer: J Urol, 1990; 144(6); 1328-30
20.. Waked R, Choucair J, Chehata N, Intravesical Bacillus Calmette-Guérin (BCG) treatment’s severe complications: A single institution review of incidence, presentation and treatment outcome: J Clin Tuberc Other Mycobact Dis, 2020; 19; 100149
21.. Gontero P, Bohle A, Malmstrom PU, The role of bacillus Calmette-Guérin in the treatment of non-muscle-invasive bladder cancer: Eur Urol, 2010; 57(3); 410-29
22.. Demers V, Pelsser V, “BCGitis”: A rare case of tuberculous epididymo-orchitis following intravesical Bacillus Calmette-Guérin therapy: J Radiol Case Rep, 2012; 6(11); 16-21
23.. de Saint Martin L, Boiron C, Poveda JD, Herreman G, [Generalized BCG infection after intravesical instillations of Calmette-Guerin bacillus.]: Presse Med, 1993; 22(29); 1352-56 [in French]
24.. Attou R, Albrich T, Kadou J, Favorable outcome in a patient with systemic BCGitis after intra-bladder instillation of Calmette-Guerin bacillus highlighting the importance of making the correct diagnosis in this rare form of sepsis: J Transl Int Med, 2019; 7(1); 34-38
25.. Kumar N, Kedarisetty CK, Kumar S, Antitubercular therapy in patients with cirrhosis: Challenges and options: World J Gastroenterol, 2014; 20(19); 5760-72
26.. Marciano BE, Huang CY, Joshi G, BCG vaccination in patients with severe combined immunodeficiency: Complications, risks, and vaccination policies [published correction appears in J Allergy Clin Immunol. 2014;134(1):244]: J Allergy Clin Immunol, 2014; 133(4); 1134-41
27.. Liu Y, Lu J, Huang Y, Ma L, Clinical spectrum of complications induced by intravesical immunotherapy of bacillus Calmette-Guérin for bladder cancer: J Oncol, 2019; 2019; 6230409
28.. Viallard JF, Denis D, Texier-Maugein J, Disseminated infection after bacille Calmette-Guérin instillation for treatment of bladder carcinoma: Clin Infect Dis, 1999; 29(2); 451-52
29.. Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: Incidence, risk factors, and outcome in a single-institution series and review of the literature: Medicine (Baltimore), 2014; 93(17); 236-54
30.. Yossepowitch O, Eggener SE, Bochner BH, Safety and efficacy of intravesical bacillus Calmette-Guerin instillations in steroid treated and immunocompromised patients: J Urol, 2006; 176(2); 482-85
31.. Izes JK, Bihrle W, Thomas CB, Corticosteroid-associated fatal myco-bacterial sepsis occurring 3 years after instillation of intravesical bacillus Calmette-Guerin: J Urol, 1993; 150(5 Pt 1); 1498-500
32.. Hanna L, Ubee SS, Boddy J, Cooke PW, Efficacy and complications of intravesical BCG in immunocompromised patients: BJU Int, 2014; 113(5); 691-93
33.. Herr HW, Dalbagni G, Intravesical bacille Calmette-Guérin (BCG) in immunologically compromised patients with bladder cancer: BJU Int, 2013; 111(6); 984-87
34.. Jenne CN, Kubes P, Immune surveillance by the liver: Nat Immunol, 2013; 14(10); 996-1006
35.. Rimola A, Soto R, Bory F, Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis: Hepatology, 1984; 4(1); 53-58
36.. Albillos A, Lario M, Álvarez-Mon M, Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance: J Hepatol, 2014; 61(6); 1385-96
37.. Dhiman RK, Saraswat VA, Rajekar H, A guide to the management of tuberculosis in patients with chronic liver disease: J Clin Exp Hepatol, 2012; 2(3); 260-70
38.. Whittaker JA, Bentley DP, Melville-Jones GR, Slater AJ, Granuloma formation in patients receiving BCG immunotherapy: J Clin Pathol, 1976; 29(8); 693-97
39.. Hogan LH, Markofski W, Bock A: Infect Immun, 2001; 69(4); 2596-603
40.. Mahairas GG, Sabo PJ, Hickey MJ: J Bacteriol, 1996; 178(5); 1274-82
41.. Metushi I, Uetrecht J, Phillips E, Mechanism of isoniazid-induced hepatotoxicity: then and now: Br J Clin Pharmacol, 2016; 81(6); 1030-36
42.. Hassan HM, Guo HL, Yousef BA, Hepatotoxicity mechanisms of isoniazid: A mini-review: J Appl Toxicol, 2015; 35(12); 1427-32
43.. Nahid P, Dorman SE, Alipanah N, Official American Thoracic Society/ Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of drug-susceptible tuberculosis: Clin Infect Dis, 2016; 63(7); e147-95
44.. Hayashi PH, Fontana RJ, Chalasani NP, Under-reporting and poor adherence to monitoring guidelines for severe cases of isoniazid hepatotoxicity: Clin Gastroenterol Hepatol, 2015; 13(9); 1676-82.e1
45.. Senousy BE, Belal SI, Draganov PV, Hepatotoxic effects of therapies for tuberculosis: Nat Rev Gastroenterol Hepatol, 2010; 7(10); 543-56
46.. Byrne JA, Strautnieks SS, Mieli-Vergani G, The human bile salt export pump: characterization of substrate specificity and identification of inhibitors: Gastroenterology, 2002; 123(5); 1649-58
47.. Steele MA, Burk RF, DesPrez RM, Toxic hepatitis with isoniazid and rifampin. A meta-analysis: Chest, 1991; 99(2); 465-71
48.. Park WB, Kim W, Lee KL, Antituberculosis drug-induced liver injury in chronic hepatitis and cirrhosis: J Infect, 2010; 61(4); 323-29
49.. Chew R, Jongbloed S, Jegatheesan D, Ethambutol is cleared by a contemporary high-flux hemodialyzer, and drug monitoring ensures safety and therapeutic effect: Antimicrob Agents Chemother, 2017; 61(6); e01988-16
50.. : Treatment of tuberculosis: Guidelines, 2010, Geneva, WHO (WHO/HTM/TB/2009.420). Available from: http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf
51.. Dhiman RK, Saraswat VA, Rajekar H, A guide to the management of tuberculosis in patients with chronic liver disease: J Clin Exp Hepatol, 2012; 2(3); 260-70
52.. Sharma P, Tyagi P, Singla V, Clinical and biochemical profile of tuberculosis in patients with liver cirrhosis: J Clin Exp Hepatol, 2015; 5(1); 8-13
53.. Alshammari TM, Larrat EP, Morrill HJ, Risk of hepatotoxicity associated with fluoroquinolones: A national case-control safety study: Am J Health Syst Pharm, 2014; 71(1); 37-43
54.. Ho CC, Chen YC, Hu FC, Safety of fluoroquinolone use in patients with hepatotoxicity induced by anti-tuberculosis regimens: Clin Infect Dis, 2009; 48(11); 1526-33
55.. Paterson JM, Mamdani MM, Manno M, Juurlink DN, Fluoroquinolone therapy and idiosyncratic acute liver injury: A population-based study: CMAJ, 2012; 184(14); 1565-70
56.. Carbon C, Comparison of side effects of levofloxacin versus other fluoroquinolones: Chemotherapy, 2001; 47(Suppl. 3); 9-48
57.. Cone C, Horowitz B, Convulsions associated with moxifloxacin: Am J Health Syst Pharm, 2015; 72(11); 910-12
58.. Wang JY, Li X, Chen JY, Tong B: Emerg Infect Dis, 2020; 26(11); 2725-27
59.. Qiao L, Cui X, Li Y, Status epilepticus attributed to moxifloxacin in an adolescent patient with spina bifida occulta: Eur J Clin Pharmacol, 2011; 67(1); 103-4
60.. Bernatowska EA, Wolska-Kusnierz B, Pac M, Disseminated bacillus Calmette-Guérin infection and immunodeficiency: Emerg Infect Dis, 2007; 13(5); 799-801
61.. Fekrvand S, Yazdani R, Olbrich P, Primary immunodeficiency diseases and bacillus Calmette-Guérin (BCG)-vaccine-derived complications: A systematic review: J Allergy Clin Immunol Pract, 2020; 8(4); 1371-86
62.. Hassanzad M, Valinejadi A, Darougar S, Disseminated bacille Calmette-Guérin infection at a glance: A mini review of the literature: Adv Respir Med, 2019; 87(4); 239-42
63.. Winger BA, Foy E, Sud SR: J Pediatr Gastroenterol Nutr, 2016; 63(1); e17-19
Figures
Tables
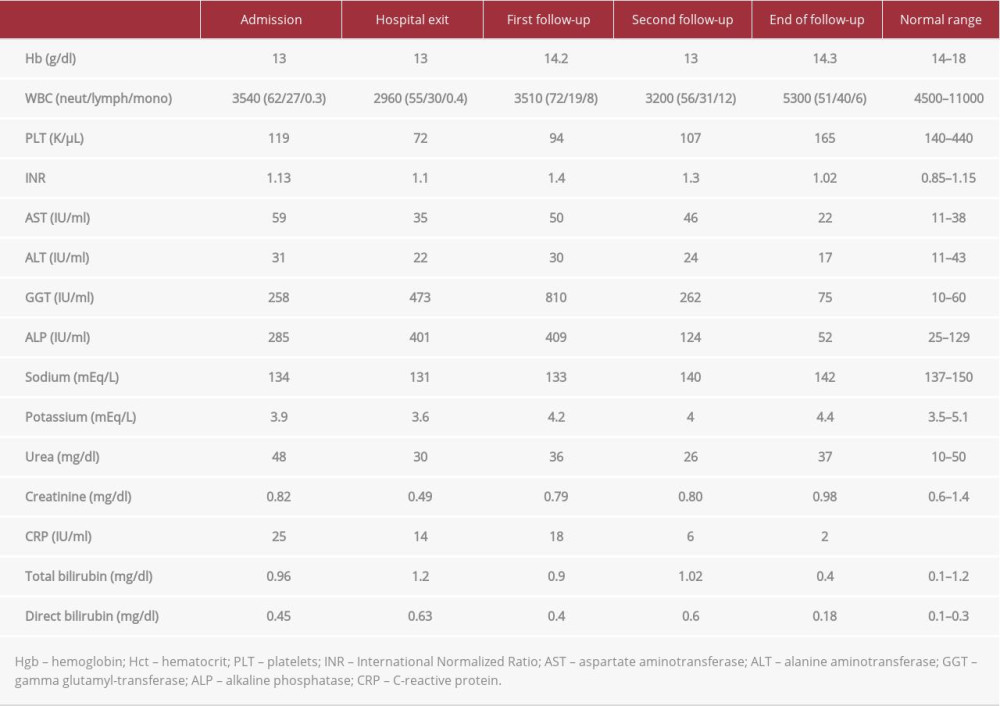 Table 1.. Laboratory results on admission and throughout the follow-up period. Time-points of follow-up as follows: “First follow-up” at one month after hospital discharge, “second follow-up” at one month after levofloxacin initiation and “end of follow-up” at 3 months after treatment completion.
Table 1.. Laboratory results on admission and throughout the follow-up period. Time-points of follow-up as follows: “First follow-up” at one month after hospital discharge, “second follow-up” at one month after levofloxacin initiation and “end of follow-up” at 3 months after treatment completion. Table 1.. Laboratory results on admission and throughout the follow-up period. Time-points of follow-up as follows: “First follow-up” at one month after hospital discharge, “second follow-up” at one month after levofloxacin initiation and “end of follow-up” at 3 months after treatment completion.
Table 1.. Laboratory results on admission and throughout the follow-up period. Time-points of follow-up as follows: “First follow-up” at one month after hospital discharge, “second follow-up” at one month after levofloxacin initiation and “end of follow-up” at 3 months after treatment completion. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








