14 May 2021: Articles 
Convexal Subarachnoid Hemorrhage Caused by Infective Endocarditis in a Patient with Advanced Human Immunodeficiency Virus (HIV): The Culprits and Bystanders
Rare disease
Faisal Khan1ABCDEF*, Neha Sharma2ABCDEF, Moin Ud Din2ABCDEF, Saloni Shirke3ABCDEF, Saima Abbas4CDEDOI: 10.12659/AJCR.931376
Am J Case Rep 2021; 22:e931376
Abstract
BACKGROUND: Convexal subarachnoid hemorrhage (cSAH), a rare form of non-aneurysmal subarachnoid hemorrhage, is confined to cerebral convexities without extension into basal cisterns or ventricles. Typical presentation includes thunderclap/progressive headache or transient focal neurological symptoms; rare manifestations include seizures, intractable vomiting, or altered mental status. Here, we report the first case of convexal subarachnoid hemorrhage and multifocal ischemic lesions caused by infective endocarditis (IE) in a treatment-naïve advanced HIV patient.
CASE REPORT: A 52-year-old HAART-naïve, HIV-positive, African American man presented with altered mental status, shortness of breath, nonproductive cough, and generalized weakness. His past medical history was significant for congestive heart failure, chronic obstructive pulmonary disease, and end-stage renal disease (noncompliant with hemodialysis). Head computed tomography (CT) showed an isolated sulcal hemorrhage in the mid-left frontal lobe. Fluid-attenuated inversion recovery/gradient recalled echo sequences confirmed a hemorrhage in the left-mid-frontal sulcus, and diffusion-weighted imaging revealed multifocal bilateral ischemic lesions. Transesophageal echocardiography exhibited mitral valve vegetations. Multifocal ischemic lesions and cSAH caused by infectious endocarditis were confirmed. Initiation of intravenous vancomycin and piperacillin-tazobactam allowed the patient to have resolution of his altered mental status. A head CT 5 days later revealed the resolution of cSAH.
CONCLUSIONS: Infective endocarditis should be considered as an underlying etiology of cSAH, especially when present with multifocal ischemic lesions. Risk factors contributing to the development of cSAH in the IE patient population should be explored in future studies. HIV has not been previously reported in this subgroup and its prevalence should be considered. The prognosis for cSAH in relation to IE is generally favorable.
Keywords: HIV, Brain Ischemia, Subarachnoid Hemorrhage, Neuroimaging, Endocarditis, HIV Infections, Magnetic Resonance Imaging
Background
Convexal subarachnoid hemorrhage (cSAH) is a rare subtype of non-aneurysmal subarachnoid hemorrhage (SAH). It is confined to the cerebral sulci, without extension into the basal cisterns or ventricles [1]. Convexal SAH has a diverse range of presenting symptoms, including thunderclap/progressive headache or transient focal neurological symptoms. Less frequently reported clinical manifestations are seizures and altered mental status [2]. A non-contrast computed tomography (CT) scan has 98% sensitivity for detecting acute SAH, which falls rapidly with time, approaching 0% at 3 weeks [3,4]. Magnetic resonance imaging (MRI) sequences including fluid-attenuated inversion recovery (FLAIR) have a sensitivity of 100% for detecting acute cSAH [3]. Additionally, gradient recall echo (GRE) sequences along with CT angiography/magnetic resonance angiography (CTA/MRA) have been shown to help determine the underlying etiology. Most of the etiologies of cSAH can be confirmed with noninvasive neurovascular imaging modalities along with appropriate systemic testing. Invasive procedures such as lumbar punctures and cerebral catheter angiography are required in equivocal cases. The prognosis in cSAH is generally better than that of aneurysmal SAH. We present a case of cSAH and multifocal ischemic lesions as a consequence of mitral valve infective endocarditis in a patient with treatment-naïve advanced HIV.
Case Report
A 52-year-old African American man with a history of treatment-naïve advanced HIV presented with shortness of breath, nonproductive cough, generalized weakness, and confusion. His past medical history was significant for hypertension, congestive heart failure, chronic obstructive pulmonary disease (COPD), and end-stage renal disease (ESRD) requiring hemodialysis via an arteriovenous fistula. He was noncompliant with hemodialysis. The patient was on disability secondary to his ESRD status, but independent for activities of daily living and instrumental activities of daily living based on the Katz index and Lawton scale, respectively. The patient denied a history of alcohol or intravenous drug use but admitted to smoking cigarettes. Aside from HIV, no prior history of sexually transmitted diseases or opportunistic infection was reported. Vital signs on admission showed blood pressure 151/87 mmHg, heart rate 133 beats/min, respiratory rate 20 breaths/min, and temperature 37.9°C. The cardiovascular exam revealed a systolic murmur 3/6 best heard at the apex, jugular venous distension, as well as bilateral lower-extremity pitting edema. The site of the arteriovenous fistula was non-tender and non-erythematous. The pulmonary exam showed symmetric expansion but bilaterally decreased breath sounds and rales. Results of the neurological examination were significant for altered mental status and slow response, consistent with hypoactive delirium. The patient opened his eyes spontaneously, was confused but able to answer questions, and obeyed commands for movement consistent with a Glasgow coma scale of 14 points. No cranial nerves, focal motor, or sensory deficits were noted.
An electrocardiogram (EKG) showed sinus tachycardia and right axis deviation without ST-segment elevation. Cardiac laboratory studies revealed a troponin level of 3.54 ng/mL that trended up to 4.04 ng/mL (0–0.4 ng/mL). A complete blood count showed hemoglobin 11.1 g/dL, white blood cell count 14.9×109/L, neutrophils 13.5 cells/Mcl, and platelets 61×109/L. A complete metabolic panel showed creatinine 15.5 mg/dL, blood urea nitrogen 110 mg/dL, estimated glomerular filtration rate (eGFR) 11 mL/min/1.73m2, albumin-creatinine ratio 167.7 mg/g, total albumin 2.6 mg/dL, lactate dehydrogenase 252 U/L, alanine aminotransferase 26 U/L, and aspartate aminotransferase 26 U/L. Other laboratory tests showed hemoglobin A1c 4.9%, CD4 count 25 cells/mm3, ferritin 34 551 ng/mL, erythrocyte sedimentation rate 78 mm/h, C-reactive protein 17.9 mg/L, brain natriuretic peptide 219 036 ng/L, and creatine kinase 849 U/L. Arterial blood gases showed pH 7.51, HCO3 of 22.8 meq/L, partial pressure of O2, and CO2 was 271 mmHg and 30 mmHg, respectively, with FiO2 and O2 saturation of 100% on a non-rebreather mask. The coagulopathy panel showed prothrombin time 15.4 s, partial thromboplastin time 46.9 s, international normalized ratio 1.35, antithrombin III 95%, lupus anticoagulant 50.4 GPL, protein C 114 IU/L, and protein S 117 IU/L. Factor V was negative, Factor VII was 28.3%, the antiphospholipid antibody syndrome panel was negative, cryoglobulins were not measured, and D-dimer was 15 717 ng/dL. A lipid panel revealed total cholesterol 101 mg/dL, triglycerides 218 mg/dL, low-density lipoprotein 40 mg/dL, and high-density lipoprotein 18 mg/dL. A urine drug screen was not available due to anuric status. The hepatitis B panel was negative. COVID-19 real-time reverse transcription-polymerase chain reaction nasopharyngeal testing was negative, and blood cultures grew methicillin-resistant
A CT scan of the head revealed a hemorrhage in the mid-left frontal lobe convexity (Figure 1). FLAIR sequences revealed hemorrhage in the left-mid-frontal lobe sulcus (Figure 2). Diffusion-weighted imaging (DWI) showed multifocal bilateral ischemic lesions in both the anterior and posterior circulation (Figures 3, 4). GRE sequence showed a convexal hemorrhagic pattern in the mid-left frontal lobe without microhemorrhages and superficial siderosis (Figure 5). Brain three-dimensional time-of-flight (3D-TOF) MRA, which has a low sensitivity of detecting vasoconstriction, did not reveal aneurysms or segmental vasoconstriction (Figures 6, 7). Transthoracic echocardiography with color Doppler showed mitral valve annular calcification, mild tricuspid regurgitation, an ejection fraction of 40% with mild left ventricular wall thickening, moderate global hypokinesis, and no regional wall abnormalities or mural thrombus. Additionally, the main pulmonary artery was found to be dilated and a small pericardial effusion was seen. There was no evidence of a right-to-left shunt secondary to patent foramen ovale or an atrial septal defect. Transesophageal echo-cardiography (TEE) exhibited 1.6×0.9 cm mobile vegetation on the anterior and posterior mitral valve leaflets, and additional vegetation was found at the tip of the central line extending into the right atrium. Telemetry monitoring during the entire hospital stay of 7 days did not reveal any episodes of cardiac arrhythmias.
A diagnosis of cSAH and multifocal ischemic lesions was made and bacterial endocarditis was determined to be the main culprit. A diagnosis of type II myocardial infarction was also suspected. Additionally, Cardiothoracic Surgery was consulted but further cardiac interventions such as coronary angiography and valvular surgery were not pursued because of the MI type and underlying comorbidities (advanced HIV, COPD, and ESRD). The patient was initiated on intravenous vancomycin and piperacillin-tazobactam and demonstrated a robust improvement in cognitive status within 24 to 48 h. Hemodialysis was resumed and a follow-up head CT performed 5 days later showed regression of the cSAH (Figure 8). The patient was discharged to a long-term acute care facility to complete the course of i.v. vancomycin for 8 weeks. He was also advised to follow-up with Cardiology for further monitoring and with Infectious Disease for initiation of HAART.
Discussion
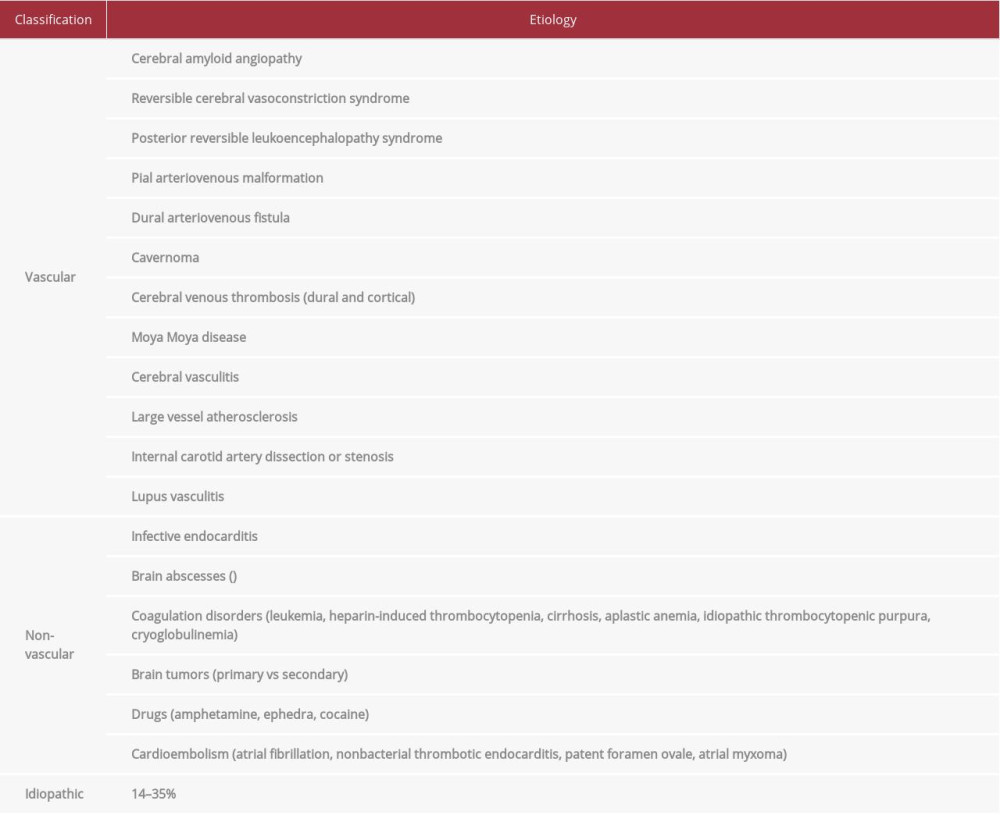
Aneurysmal subarachnoid hemorrhage (saccular, fusiform, or mycotic) is the most common etiology of atraumatic SAH [5,6]. Non-aneurysmal types, including perimesencephalic and convexal, are less prevalent (Figure 9). Diagnostic and management guidelines are well established for aneurysmal sub-types, but they are not well defined for non-aneurysmal SAH (especially cSAH). This is mostly due to its myriad etiologies (Table 1) and the lack of larger comprehensive studies.
Cerebral amyloid angiopathy (CAA) and reversible cerebral vasoconstriction syndrome (RCVS) are the most common causes of atraumatic cSAH. Cerebral amyloid angiopathy is frequently seen in patients older than 60 years, with characteristic findings on GRE including microhemorrhages and superficial siderosis. Reversible cerebral vasoconstriction syndrome is commonly seen in patients younger than 60 years and appears as segmental vasoconstriction; this can be seen on 3D TOF MRA, which, compared to conventional angiography, has an 80% sensitivity for detecting focal vasoconstriction [7]. Posterior reversible encephalopathy syndrome (PRES), seen in patients younger than age 60 years, has confluent white matter hyperintensities on FLAIR sequencing [1,5]. These characteristic findings were not seen on our patients’ neuroimaging, thus helping us exclude CAA, RCVS, and PRES as underlying etiologies of cSAH for our patient.
In the absence of common etiologies, rare causes need to be explored (Table 1). Dakay et al suggested a diagnostic approach to determine the underlying etiology of cSAH. According to their algorithm, if brain MRI shows multifocal ischemic lesions along with cSAH, endocarditis and vasculitis should be considered [8].
Our patient fulfilled the modified Duke’s criteria for infective endocarditis (IE), which is the most probable etiology for both cSAH and multifocal ischemic lesions. Multiple studies have cited IE with various implicated pathogens (
Recently, Boukobza et al conducted a large retrospective cohort study with the most recent available data on cSAH associated with IE. They identified 240 patients with IE, of which 31 (12.9%) patients had cSAH. The majority of these cSAH cases (21/31; 67.7%) were not related to an intracranial infectious aneurysm (IIA). No evidence of IIA was found in our patient on the 3D-TOF MRA. In terms of clinical presentation, Boukobza et al found that cSAH was an incidental finding in 71%, and altered mental status presented in 9.7%. Our patient presented with altered mental status, which we believe resulted from sepsis because he had a robust response to antibiotics.
Additionally, Boukobza et al also reported that most cases had a unilateral bleeding pattern localized to a single sulcus. The most commonly involved sites were the frontal lobe, followed by the parietal and temporal lobes. These patients were also found to have concurrent, acute multifocal ischemic lesions, cerebral microbleeds, and/or co-infections (ie, frontal micro-abscesses or meningitis). Furthermore,
Infective endocarditis is associated with mycotic aneurysms, presenting as a non-convexal, hemorrhagic pattern [14]. Although mycotic aneurysms can be detected with MRA or CTA, digital subtraction angiography (DSA) is the criterion standard diagnostic test [15–17]. In our case, mycotic aneurysms were not detected with MRA, and further vascular imaging, including DSA, was unfortunately declined by the patient.
Furthermore, some patients may be at an increased risk of developing cSAH with infective endocarditis. Although cardiac vegetations >15 mm and infection with
The presence of treatment-naïve, advanced HIV was a significant caveat in our case. To our knowledge, this has not been previously reported with infective endocarditis in the context of convexal subarachnoid hemorrhage and multifocal ischemic lesions. We searched PubMed and Google Scholar using the keywords “HIV,” “infective endocarditis,” and “convexal subarachnoid hemorrhage”, but we could not find any published data in the English literature citing the HIV status of patients with cSAH. We also do not have any data regarding the incidence of HIV from the study by Boukobza et al [13]. Considering the diverse range of cerebrovascular complications associated with HIV (Figure 10), future studies should investigate its association with cSAH. We suggest considering HIV as a modifier that increases the incidence of cSAH in patients with IE.
Cerebral vasculitis and aneurysmal arteriopathy are the most common forms of HIV-related cerebrovascular complications. Cerebral vasculitis accounts for 13–28% of ischemic strokes in HIV-positive patients [18]. Risk factors for developing HIV vasculitis include African American descent, CD4 count <200, and lack of highly active antiretroviral therapy (HAART), as seen in our patient [19]. A probable diagnosis of cerebral vasculitis can be made based on CT or MRI findings of ischemic lesions in more than 1 vascular territory, as seen in our patient (Figures 3, 4) [20]. For a definitive diagnosis, invasive testing, including lumbar puncture, cerebral catheter angiography (CCA), and leptomeningeal biopsy, is required. Unfortunately, our patient declined all of these procedures. Furthermore, treatment of HIV vasculitis includes the use of immunosuppressants (steroids, methotrexate, azathioprine) and biologic agents (tocilizumab), in addition to HAART [21]. Our patient experienced immediate, significant improvement with antibiotic treatment without requiring immunosuppressants or HAART, thus negating the diagnosis of HIV-related cerebral vasculitis.
HIV-related cerebral aneurysmal arteriopathy is widely reported in the literature and has been hypothesized to be caused by invasion of the intracranial vascular endothelium by HIV, thus triggering an immune response that induces turbulent blood flow, leading to thrombosis and vascular remodeling [22,23]. Repeat infections with HIV-associated opportunistic organisms increase cytokine production, thus enhancing the effect of HIV on the vasculature wall [24]. Our patient did not have any opportunistic infections and no aneurysmal pathology was found on 3-D TOF MRA imaging. Although HIV vasculitis and aneurysmal arteriopathy were not diagnosed in our patient, we do believe that HIV increased the risk of cSAH by facilitating IE and thus leading to cSAH and ischemic stroke.
We also considered other risk factors that may have contributed to cSAH in our case, including thrombocytopathy (relating to HIV or ESRD status). Thrombocytopenia is a well-known complication in HIV patients. Silvestrini et al reported a case of a HIV-positive patient diagnosed with spontaneous SAH secondary to autoimmune thrombocytopenia [25]. Although our patient had thrombocytopenia (61×109/L), the autoimmune workup was negative.
Alternatively, uremic thrombocytopathy, as a consequence of untreated ESRD, may have also played a role. Uremia increases the synthesis of nitric oxide in platelets and endothelial cells [26]. Subsequently, an increase in cyclic GMP and a decrease in thromboxane A2 and ADP levels leads to impairment in platelet aggregation [27]. Therefore, uremic thrombocytopathy could have been a contributing factor in our case. Furthermore, a retrospective cohort study in 2016 by Molnar et al found that in patients with chronic kidney disease, a declining eGFR and an increasing albumin-creatinine ratio independently increases the risk of intracranial hemorrhage by 20-fold over a period of 3 years [28].
Determining the underlying cause of cSAH is crucial, as it provides guidance for therapeutic intervention and prognostication. Establishing an etiology can be challenging when noninvasive neurovascular imaging, including MRI, CTA, and MRA, do not reveal the underlying pathology. Use of invasive diagnostic modalities such as cerebral catheter angiography (CCA), lumbar puncture, and leptomeningeal biopsy should be employed in cases where a definitive diagnosis cannot be ascertained. Dakay et al reported that noninvasive neuroimaging, including CT, MRI, CTA, and MRA, revealed the underlying etiology of cSAH in 57% of patients; this increased to 94.2% with the use of CCA. Approximately 14–35% of cases remain idiopathic [8].
Currently, no therapeutic guidelines exist for the management of cSAH. Treatment is primarily supportive and targeted towards the underlying etiology. Our patient showed regression of cSAH within 5 days of initiating antibiotics. The prognosis for patients with cSAH in conjunction with CAA (age >60 years, microhemorrhage, and white matter hyperintensities on MRI) is worse, with a higher risk of morbidity [2]. There is a higher risk of rebleeding with RCVS in ~10% of patients [29]. Boukobza et al did not observe any recurrences of cSAH and noted favorable outcomes in patients with IE [13]. Prognostic data for other etiologies of cSAH are not available. Therefore, additional larger-scale studies are needed to enhance our understanding of the prognostic markers of cSAH. Identification of additional risk factors is the first step, and preventive therapy targeted towards contributing factors will result in a favorable prognosis.
We acknowledge that our case report has certain limitations. Diagnostic procedures, such as cerebral catheter angiography and lumbar puncture, can detect occult aneurysms or vasculitis and infectious etiologies, respectively. These tests were not performed since the patient refused invasive procedures. The rapid and significant clinical improvement with intravenous antibiotics negated the utility of these diagnostic tests. Our patient had multifocal ischemic lesions and cSAH localized to a single sulcus (frontal region) with valvular vegetations measuring >15 mm, similar to the case reported by Boukobza et al. All of these factors led us to determine that infective endocarditis was the underlying etiology of cSAH and multifocal ischemic lesions in our patient.
Conclusions
Convexal subarachnoid hemorrhage has been associated with multiple well-established etiologies. Clinicians should consider infective endocarditis as an underlying etiology of cSAH, particularly in the presence of multifocal ischemic lesions and relevant systemic features. The presence of additional risk factors in patients with IE may contribute to the development of cSAH. Here, we reported the first case of a patient with a history of treatment-naïve advanced HIV, who suffered from convexal subarachnoid hemorrhage and multifocal ischemic lesions, secondary to infective endocarditis. The prevalence of HIV has not been previously reported in the IE subgroup and its role in the pathogenesis of cSAH should be considered in future studies.
Figures
References:
1.. Kumar S, Goddeau RP, Selim MH, Atraumatic convexal subarachnoid hemorrhage: Clinical presentation, imaging patterns, and etiologies: Neurology, 2010; 74(11); 893-99
2.. Beitzke M, Gattringer T, Enzinger C, Clinical presentation, etiology, and long-term prognosis in patients with nontraumatic convexal subarachnoid hemorrhage: Stroke, 2011; 42(11); 3055-60
3.. Renou P, Tourdias T, Fleury O, Debruxelles S, Atraumatic nonaneurysmal sulcal subarachnoid hemorrhages: A diagnostic workup based on a case series: Cerebrovasc Dis, 2012; 34(2); 147-52
4.. Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA, Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis: Stroke, 2016; 47(3); 750-55
5.. Cuvinciuc V, Viguier A, Calviere L, Isolated acute nontraumatic cortical subarachnoid hemorrhage: Am J Neuroradiol, 2010; 31(8); 1355-62
6.. Shimizu T, Takeda N, Takahashi M, Subarachnoid hemorrhage from mycotic aneurysms: Intern Med, 2006; 45(20); 1189-90
7.. Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D, Reversible cerebral vasoconstriction syndrome, Part 2: Diagnostic work-up, imaging evaluation, and differential diagnosis: Am J Neuroradiol, 2015; 36(9); 1580-88
8.. Dakay K, Mahta A, Rao S, Yield of diagnostic imaging in atraumatic convexity subarachnoid hemorrhage: J Neurointerv Surg, 2019; 11(12); 1222-26
9.. Chukwudelunzu FE, Brown RD, Wijdicks EF, Steckelberg JM, Subarachnoid haemorrhage associated with infectious endocarditis: Case report and literature review: Eur J Neurol, 2002; 9(4); 423-27
10.. Graff-Radford J, Fugate JE, Klaas J, Distinguishing clinical and radiological features of non-traumatic convexal subarachnoid hemorrhage: Eur J Neurol, 2016; 23(5); 839-46
11.. Boukobza M, Smaali I, Duval X, Laissy JP, Convexity subarachnoid hemorrhage, pseudomonas aeruginosa (PA) infective endocarditis and left atrial appendage occluder (LAAO) device infection: A case report. Open Neuroimag J, 2017; 11; 26-31
12.. Krapf H, Skalej M, Voigt K, Subarachnoid hemorrhage due to septic embolic infarction in infective endocarditis: Cerebrovasc Dis, 1999; 9(3); 182-84
13.. Boukobza M, Ilic-Habensus E, Duval X, Laissy JP, Acute convexity subarachnoid hemorrhage (cSAH) in infectious endocarditis (IE): imaging features and follow-up: J Neurol, 2020; 267(10); 2971-82
14.. Fisk M, Peck LF, Miyagi K, Mycotic aneurysms: A case report, clinical review, and novel imaging strategy: QJM, 2012; 105(2); 181-88
15.. González I, Sarriá C, López J, Symptomatic peripheral mycotic aneurysms due to infective endocarditis: A contemporary profile: Medicine (Baltimore), 2014; 93(1); 42-52
16.. Hui FK, Bain M, Obuchowski NA, Mycotic aneurysm detection rates with cerebral angiography in patients with infective endocarditis: J Neurointerv Surg, 2014; 7(6); 449-52
17.. Walkoff L, Brinjikji W, Rouchaud A, Comparing magnetic resonance angiography (MRA) and computed tomography angiography (CTA) with conventional angiography in the detection of distal territory cerebral mycotic and oncotic aneurysms: Interv Neuroradiol, 2016; 22(5); 524-28
18.. Cheron J, Wyndham-Thomas C, Sadeghi N, Naeije G, Response of human immunodeficiency virus-associated cerebral angiitis to the combined anti-retroviral therapy: Front Neurol, 2017; 8; 95
19.. Thawani JP, Nayak NR, Pisapia JM, Aneurysmal vasculopathy in human-acquired immunodeficiency virus-infected adults: Imaging case series and review of the literature: Interv Neuroradiol, 2015; 21(4); 441-50
20.. Benjamin LA, Bryer A, Lucas S, Arterial ischemic stroke in HIV: Defining and classifying etiology for research studies: Neurol Neuroimmunol Neuroinflamm, 2016; 3(4); e254
21.. Vega LE, Espinoza LR, Vasculitides in HIV infection: Curr Rheumatol Rep, 2020; 22(10); 60
22.. Ake JA, Erickson JC, Lowry KJ, Cerebral aneurysmal arteriopathy associated with HIV infection in an adult: Clin Infect Dis, 2006; 43(5); e46-50
23.. Blignaut G, Loggenberg E, De Vries C, The radiological appearance of intracranial aneurysms in adults infected with the human immunodeficiency virus (HIV): South African Journal of Radiology, 2014; 18(1) Art. #586
24.. Tipping B, de Villiers L, Candy S, Wainwright H, Stroke caused by human immunodeficiency virus-associated intracranial large-vessel aneurysmal vasculopathy: Arch Neurol, 2006; 63(11); 1640-42
25.. Silvestrini M, Floris R, Tagliati M, Spontaneous subarachnoid hemorrhage in an HIV patient: Ital J Neurol Sci, 1990; 11(5); 493-95
26.. Noris M, Benigni A, Boccardo P, Enhanced nitric oxide synthesis in uremia: Implications for platelet dysfunction and dialysis hypotension: Kidney Int, 1993; 44(2); 445-50
27.. Hedges SJ, Dehoney SB, Hooper JS, Evidence-based treatment recommendations for uremic bleeding: Nat Clin Pract Nephrol, 2007; 3(3); 138-53
28.. Molnar AO, Bota SE, Garg AX, The risk of major hemorrhage with CKD: J Am Soc Nephrol, 2016; 27(9); 2825-32
29.. Forman R, Conners JJ, Song SY, The spectrum of nontraumatic convexity subarachnoid hemorrhage: J Stroke Cerebrovasc Dis, 2019; 28(12); 104473
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250