23 May 2023: Articles 
A Case of an 82-Year-Old Man with a Spinal Extradural Malignant Ossifying Fibromyxoid Tumor
Challenging differential diagnosis, Rare disease
Qiqi Lu1BCDEF, Chi Long Ho2345ABCDEFGDOI: 10.12659/AJCR.939408
Am J Case Rep 2023; 24:e939408
Abstract
BACKGROUND: Ossifying fibromyxoid tumor (OFMT) is a rare mesenchymal tumor predominantly involving the subcutaneous tissues or skeletal muscles in the proximal extremities, typically in middle-aged men. OFMT in the spine is extremely rare, with only 3 previously reported cases in the literature.
CASE REPORT: Here, we present a rare case of an 82-year-old man presenting with paresthesia of both arms and weakness of both legs, who underwent magnetic resonance imaging (MRI) of the spine, which showed an aggressive extradural tumor. Following surgical debulking, histology examination revealed a tumor of stromal origin with myxoid and ossifying components and pleomorphic features. Overall findings were suggestive of a malignant OFMT. The patient underwent postoperative adjuvant radiotherapy. However, the first follow-up MRI study at 8 months showed residual tumor, which also demonstrated avid tracer uptake on technetium-99m scintigraphy and PET-CT scans. The second MRI follow-up about 9 months later showed several metastatic foci along the craniospinal axis. Despite subsequent resection of the spinal metastasis, the patient eventually died of sepsis about 21 months after the initial tumor diagnosis.
CONCLUSIONS: We presented a case of extradural spinal malignant OFMT and highlighted the difficulty distinguishing this rare primary tumor from spinal metastases. In this case, MRI signal intensities and identifying intratumoral bone formation, combined with histopathology following surgical resection, confirmed the diagnosis. This case has also shown the importance of follow-up by a multidisciplinary team to monitor for the recurrence of primary OFMT.
Keywords: Diagnostic Imaging, Soft Tissue Neoplasms, Pathology, Male, Middle Aged, Humans, Aged, 80 and over, Positron Emission Tomography Computed Tomography, Fibroma, Sarcoma, Magnetic Resonance Imaging, Follow-Up Studies
Background
Ossifying fibromyxoid tumor (OFMT) is a rare mesenchymal tumor of the soft tissues, first described by Enzinger et al in 1989 [1]. Although they are often located within the subcutaneous tissues, they can affect the deep muscles and bones [2,3]. They present clinically as a slowly enlarging painless mass in the subcutaneous tissues or skeletal muscles [2,3]. They commonly arise in the proximal extremities [2,3] but their occurrence at other sites, including the head, neck, and torso have also been reported [4–6]. It is exceedingly rare for OFMT to involve the spine. Typical demographics of OFMTs involve middle-aged men and, less commonly, the elderly [2]. In this report, we present a rare case of an 82-year-old elderly man with back pain, upper-limb paresthesia, and lower-limb weakness, who was subsequently diagnosed with malignant extradural OFMT in the cervical spine. The imaging and histopathological characteristics, differential diagnosis, treatment, and prognosis of this rare tumor are discussed in detail in this report.
Case Report
An 82-year-old man of Chinese ethnicity presented to the Emergency Department with progressively worsening upper back pain, upper-limb paresthesia, and unsteady gait for 1-week duration. His history was remarkable for gastritis, hyperplastic intestinal polyps, and intestinal metaplasia. Neurological examination revealed bilateral upper-limb paresthesia and weakness of both legs, with a motor power of 3/5.
Initial magnetic resonance imaging (MRI) of the cervical spine revealed an abnormal high T2 signal lesion with post-contrast enhancement centered at C7-T1 level with an intraspinal soft-tissue mass that spread from C4 to T2 levels with extension into the left neural exit canals (Figure 1). As the spinal tumor formed an obtuse angle with the surrounding CSF and dura, it was situated in the extradural location. The extradural tumor compressed the spinal cord (Figure 1). Furthermore, there was vertebral marrow infiltration and enhancement of the T1 vertebral body and left pedicle. Axial CT neck across the center of the tumor at C6 level showed erosions of the posterior elements and faint intratumoral calcification (Figure 2). Subsequently, the patient underwent C5-T2 laminectomy and subtotal tumor resection/debulking and posterior translaminar spinal fixation with instrumentation.
Histology examination of the excised tumor samples showed a well-circumscribed mesenchymal tumor with uniformly round-to-ovoid-shaped cells arranged in clusters within a myxoid and hyalinized stroma. There was scattered nuclear atypia with high mitotic activity with an osseous fibrous rim and thick collagenous capsule (Figure 3). Immunohistochemistry showed that the tumor was strongly positive for vimentin and patchily positive for EMA, CD99, and AE 1/3. There was a high Ki67 proliferation index of 40–50%. The findings were compatible with a malignant ossifying fibromyxoid tumor FNCLCC (Federation Nationale des Centres de Lutte Contre le Cancer) grade 2 (Figure 3).
Postoperative local radiation therapy (RT) of 30 Gy in 10 fractions was administered 6 weeks after surgery. The first follow-up MRI at 8 months after surgery showed a remnant extradural tumor from C6 to T1 levels with extension into the left C6-7 and C7-T1 neural exit foramina and left paravertebral region (Figure 4). Positron emission tomographic-computed tomography (PET-CT) scans at C7 level showed focal raised 18F-FDG up-take at the anteromedial aspect of the C7 vertebra with maximum standardized uptake values and SUVmax=25, indicating residual tumor, while a faint focal FDG uptake in the posterior aspect of the conus medullaris (SUVmax=3.5) was deemed indeterminate for metastasis at the time of scanning (Figure 4).
In the second follow-up MRI about 9 months later, the previously noted faint focus of raised FDG uptake at the conus medullaris turned out to be a drop metastasis with leptomeningeal spread to bilateral exiting L2 nerve roots (Figure 5). There were new dural-based enhancing lesions along the petrous temporal bones at bilateral cerebellopontine angles, which were suspected of metastasis (Figure 5). The patient subsequently underwent L1-L2 laminectomy of the metastatic lesion at the conus medullaris. However, he developed gram-negative bacteremia secondary to a prostatic abscess about 3 months later and eventually succumbed to his illness and died 21 months after the initial tumor surgery.
Discussion
OFMTs are rare tumors, particularly in the spinal region. Although non-specific, some imaging features, such as intratumoral ossification, hemorrhage foci and avid FDG uptake secondary to ossification, may provide evidence of the presence of a malignant spinal tumor. Histology is still the criterion standard for diagnosis. These malignant tumors are known for having a high risk of local recurrence and metastasis. The mainstay treatment is surgery with postoperative radiotherapy, as shown in this case.
OFMT can affect a wide range of age groups, predominantly involving those in the fifth and sixth decades of life with a mean age of 51 years [7]. It has a slight male predilection, with a male-to-female ratio of 1.5: 1 [7]. The tumors typically present as soft-tissue masses within the subcutis or deep soft tissues, including the skeletal muscles [2,3]. It is exceedingly rare for the spine to be affected by OFMT, with only 4 reported cases thus far in the literature (Table 1). The patients with spinal involvement were often asymptomatic or insidious until the late stage, with neurological deficits involving the extremities. These tumors are predominantly extradural in location, and typically show an infiltrative growth pattern with spinal cord compression, with the potential for local recurrence [8,9]. Unlike the previously reported OFMT cases, our patient showed distant metastasis from the cervical to the lumbar spine and cerebellopontine angles. This indicates the potential for malignant transformation of OFMTs with dissemination along the craniospinal axis via the cerebrospinal fluid (CSF). Locoregionally, OFMTs often present as a nodular soft-tissue mass, with a peripheral rim of ossification or “bone shell” seen in 60–70% of cases [3]. They may present as a mass with an incomplete rim of ossification, and, less often, central ossification. The underlying bones can be eroded with or without periosteal reactions [3].
CT scans typically show soft-tissue mass, with peripheral or central (incomplete) ossification in nearly 70% of the cases [1,4,7] (Figure 2). Given its exceedingly rare spinal involvement, there is a paucity of imaging data on spinal OFMT. On MRI of the spine, the tumor typically demonstrates isointensity to the muscles on T1-weighted images (WI) and intermediate-to-high signal on T2WI (Figure 1) [8]. These features are also seen in our case and another paraspinal OFMT reported by Cha et al [10]. Occasionally, the tumor demonstrates areas of high T1-and T2-weighted signal intensities, indicating intratumoral hemorrhage [8] but these are not observed in our patient. In our case, the extradural nature of the OFMT formed an obtuse angle with the surrounding CSF space, distinguishing it from intradural extramedullary tumors, which often form an acute angle with the surrounding CSF.Technetium-99m methylene diphosphonate scintigraphy may demonstrate avid tracer uptake due to mature bone formation within the tumor [6]. Likewise, there is an osseous fibrous rim within a thick collagenous capsule identified on microscopic examination of the tumor specimens, as in our case. Intratumoral mature bone is, however, absent in approximately one-third of the cases [9]. Positron electron tomographic (PET)-CT study can be employed to detect recurrent tumor or metastasis following initial tumor debulking, particularly when remnant tumor is suspected. On microscopic examination, OFMT often shows variable histopathologic features containing bone and osteoid and collagen elements [9]. Most tumors are composed of small, evenly sized, round tumor cells separated by variable amounts of osteoid [6,9,11,12]. According to Folpe et al, OFMT has a high nuclear grade with a high mitotic activity of >2 mitotic figures per 50 high-power fields [13]. Immunohistochemically, they showed expression of S-100 protein, CD99 (MIC2), CD56, and neuron-specific enolase [5,11,12,14]. Other positive neural markers of OFMT are Leu-7, GFAP, neurofilament, synaptophysin, and collagen type IV [11]. Macroscopically, most tumors are surrounded by a thick collagenous capsule and osseous fibrous rim [6,11]. In 80% of the cases, the tumor periphery consists of an incomplete shell of bone [14].
Having histologic evidence of mature lamellar bone formation, OFMTs should be differentiated from non-neoplastic conditions, including chronic organizing hematomas with calcification and myositis ossificans. Other epidural and paraspinal tumors including epithelioid nerve sheath and smooth muscle tumors [15], myxoid chondrosarcoma, and chondroid syringoma should also be differentiated from OFMT [16]. Unlike OFMTs, the aforementioned tumors often present with high T2 signal content, restricted diffusion, and lack of intratumoral calcification.
Although metastases are the most common malignant condition in the spine, OFMTs should be differentiated from other common malignant epidural spinal tumors, including multiple myeloma, lymphoma, chordoma, chondrosarcoma, osteosarcoma, giant cell tumor, and myeloid sarcoma [7]. The imaging features of the common malignant tumors found in the extradural space are summarized in Table 2.
Surgical resection is the mainstay treatment, including tumor debulking [14]. Once the diagnosis of OFMT is made, further evaluation of the potential tumor metastasis is essential.
Adjuvant treatment with postoperative radiotherapy [5] is considered after piecemeal surgical resection, debulking, or margin-positive surgical resections to reduce the risk of local recurrence. Recurrent tumor always requires re-operation and resection.
Having high nuclear grade with high mitotic activity, OFMTs are considered tumors with intermediate-to-high risk of malignancy, with potential for local invasion, recurrence, and distant metastasis [13]. Among the reported spinal OFMT cases, local invasion and tumor recurrence were detected in 3 of the 4 reported cases despite adequate surgery with or without adjuvant chemo- or radiotherapy [8–10]. These suggest that surgical resection with routine postoperative adjuvant therapies may not be sufficient to control malignant spinal OFMTs.
Besides recurrence, our case with malignant OFMT presented with distant metastases along the craniospinal axis. Although not unexpected, such an extent of craniospinal spread of disease has never been reported in previous spinal OFMT cases.
Conclusions
Owing to its rare presentation in the spine, OFMT is often under-recognized. It should be differentiated from other epidural and paraspinal spinal tumors besides the more common metastases and malignant primary tumors in the spine (Table 2). On MRI, OFMTs typically show low-to-isointense T1 and intermediate-to-high T2 signal intensities. Owing to the propensity for intratumoral bone formation, this malignant tumor often shows intralesional calcifications and avid tracer uptake on technetium-99m scintigraphy and PET-CT scans. Surgical resection is the mainstay treatment with postoperative adjuvant radiotherapy. Multidisciplinary approaches with close postoperative surveillance are essential in management given the high risk of local tumor recurrence and distant metastases.
Figures
References:
1.. Enzinger FM, Weiss SW, Liang CY, Ossifying fibromyxoid tumor of soft parts. A clinicopathological analysis of 59 cases: Am J Surg Pathol, 1989; 13(10); 817-27
2.. Schneider N, Fisher C, Thway K, Ossifying fibromyxoid tumor: Morphology, genetics, and differential diagnosis: Ann Diagn Pathol, 2016; 20; 52-58
3.. Bakiratharajan D, Rekhi B, Ossifying fibromyxoid tumor: An update: Arch Pathol Lab Med, 2016; 140(4); 371-75
4.. Sharma K, Hughes D, Harper RD, Ossifying fibromyxoid tumor (OFMT) – a rare cause of a painful thumb: Int J Surg Case Rep, 2015; 7C; 93-95
5.. Velasco IA, Zhang R, Li T, Wang D, Ossifying Fibromyxoid tumor of soft parts in head and neck: Case report and literature review: Diagn Pathol, 2018; 13(1); 21
6.. Namiki T, Hsieh M, Iwamoto Y, Subcutaneous ossifying fibromyxoid tumor of the scalp: A potential importance of CT, MRI, and PET/CT on the diagnosis: Int J Dermatol, 2019; 58(6); e121-e23
7.. Carter CS, Patel RM, Ossifying fibromyxoid tumor: A review with emphasis on recent molecular advances and differential diagnosis: Arch Pathol Lab Med, 2019; 143(12); 1504-12
8.. Sangala JR, Park P, Blaivas M, Lamarca F, Paraspinal malignant ossifying fibromyxoid tumor with spinal involvement: J Clin Neurosci, 2010; 17(12); 1592-94
9.. De Wandeler T, Van Gestel D, Jissendi Tchofo P, Ossifying fibromyxoid tumor with spinal cord compression and epiduritis: J Belg Soc Radiol, 2019; 103(1); 18
10.. Cha JH, Kwon JW, Cho EY, Ossifying fibromyxoid tumor invading the spine: A case report and review of the literature: Skeletal Radiol, 2008; 37(12); 1137-40
11.. Miliaras D, Meditskou S, Ketikidou M, Ossifying fibromyxoid tumor may express CD56 and CD99: A case report: Int J Surg Pathol, 2007; 15(4); 437-40
12.. Hirose T, Shimada S, Tani T, Hasegawa T, Ossifying fibromyxoid tumor: Invariable ultrastructural features and diverse immunophenotypic expression: Ultrastruct Pathol, 2007; 31(3); 233-39
13.. Folpe AL, Weiss SW, Ossifying fibromyxoid tumor of soft parts: A clinicopathologic study of 70 cases with emphasis on atypical and malignant variants: Am J Surg Pathol, 2003; 27(4); 421-31
14.. Miettinen M, Finnell V, Fetsch JF, Ossifying fibromyxoid tumor of soft parts – a clinicopathologic and immunohistochemical study of 104 cases with long-term follow-up and a critical review of the literature: Am J Surg Pathol, 2008; 32(7); 996-1005
15.. Garner HW, Wilke BK, Fritchie K, Bestic JM, Epithelioid schwannoma: Imaging findings on radiographs, MRI, and ultrasound: Skeletal Radiol, 2019; 48(11); 1815-20
16.. Kerimoglu U, Aydingoz U, Ozkaya O, MRI of a benign chondroid syringoma: Br J Radiol, 2006; 79(944); e59-e61
17.. Avni B, Koren-Michowitz M, Myeloid sarcoma: Current approach and therapeutic options: Ther Adv Hematol, 2011; 2(5); 309-16
18.. Majithia N, Rajkumar SV, Lacy MQ, Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents: Leukemia, 2016; 30(11); 2208-13
19.. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N, Hemangiopericytoma: Long-term outcome revisited: J Neurosurg, 2011; 114(3); 747-55
20.. Rohatgi S, Ramaiya NH, Jagannathan JP, Metastatic chordoma: Report of the two cases and review of the literature: Eurasian J Med, 2015; 47(2); 151-54
21.. Fromm J, Klein A, Baur-Melnyk A, Survival and prognostic factors in conventional central chondrosarcoma: BMC Cancer, 2018; 18(1); 849
22.. Halldorsson A, Brooks S, Montgomery S, Graham S, Lung metastasis 21 years after initial diagnosis of osteosarcoma: A case report: J Med Case Rep, 2009; 3; 9298
23.. Viswanathan S, Jambhekar NA, Metastatic giant cell tumor of bone: Are there associated factors and best treatment modalities?: Clin Orthop Relat Res, 2010; 468(3); 827-33
Figures
Tables
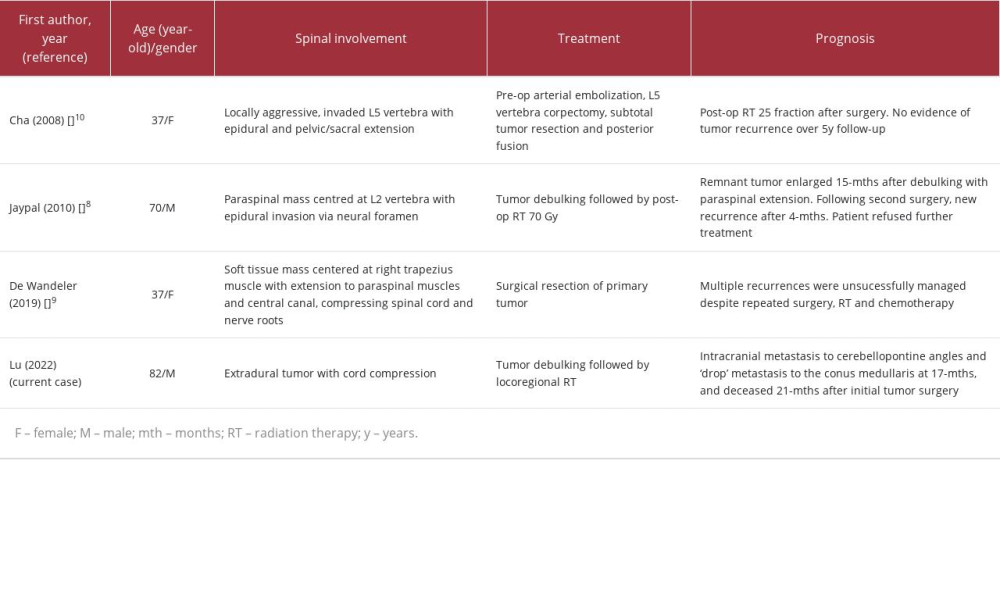 Table 1.. Summary of previous cases of spinal ossifying fibromyxoid tumors (OFMT) reported in the literature.
Table 1.. Summary of previous cases of spinal ossifying fibromyxoid tumors (OFMT) reported in the literature.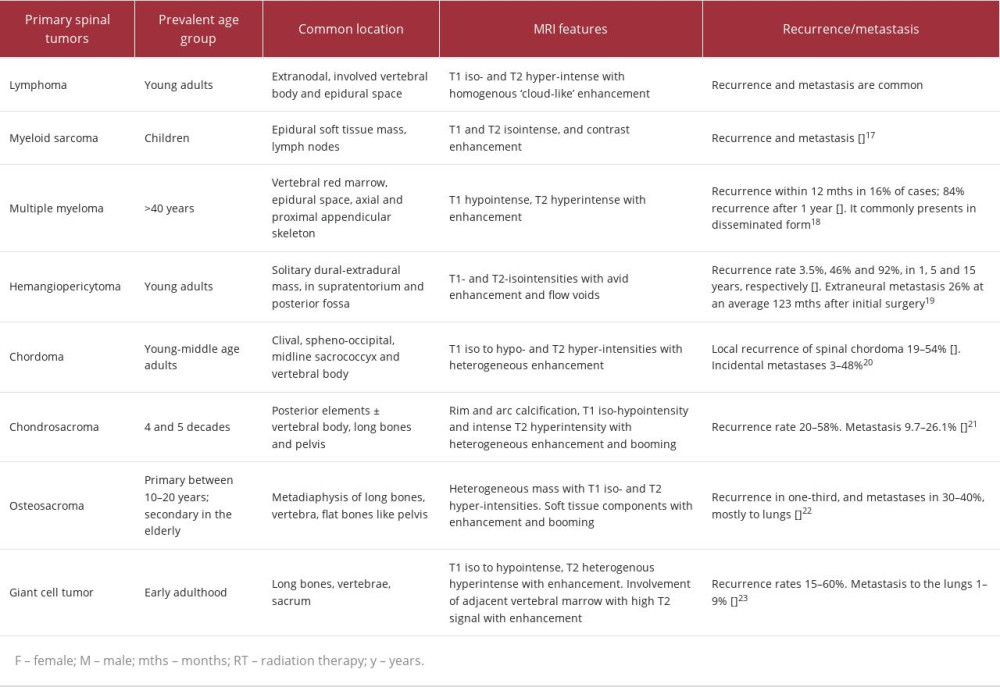 Table 2.. Summary of common primary spinal extradural tumors.
Table 2.. Summary of common primary spinal extradural tumors. Table 1.. Summary of previous cases of spinal ossifying fibromyxoid tumors (OFMT) reported in the literature.
Table 1.. Summary of previous cases of spinal ossifying fibromyxoid tumors (OFMT) reported in the literature. Table 2.. Summary of common primary spinal extradural tumors.
Table 2.. Summary of common primary spinal extradural tumors. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








