18 August 2023: Articles 
IgG4-Related Membranous Nephropathy with Acute Nephrotic Syndrome During Successful Steroid Maintenance Treatment for Type 1 Autoimmune Pancreatitis
Unusual clinical course, Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Hiroshi Ito12ABCDEF, Kenji AshidaDOI: 10.12659/AJCR.940707
Am J Case Rep 2023; 24:e940707
Abstract
BACKGROUND: Immunoglobulin G4 (IgG4)-related diseases (IgG4-RD) are systemic fibroinflammatory diseases that can develop asynchronously in multiple organs. IgG4-related kidney disease (IgG4-RKD) is generally characterized by tubulointerstitial nephritis but can also manifest as membranous nephropathy without tubulointerstitial nephritis. IgG4-related membranous nephropathy can present as a phenotype of systemic disorders, including autoimmune pancreatitis-associated diabetes mellitus; however, its clinical features remain unclear.
CASE REPORT: A 56-year-old Japanese man presented to our university hospital with bilateral edema of his lower legs. He had received a diagnosis of type 1 autoimmune pancreatitis and associated diabetes mellitus 16 months prior. He was successfully treated with oral glucocorticoids 25 mg/day of prednisolone as an initial dose, followed by titration down to a maintenance dose (5 mg/day), without recurrence of autoimmune pancreatitis. The pancreas showed atrophy and required basal-bolus insulin therapy owing to insulin insufficiency. Massive proteinuria and hypoalbuminemia with nephrotic syndrome on examination led to a renal biopsy to investigate the etiology and diagnosis of IgG4-RKD. Methylprednisolone and cyclosporine A were successfully administered to ameliorate the proteinuria and control systemic IgG4-RD with IgG4-related membranous nephropathy.
CONCLUSIONS: Ig4-RKD occurred despite maintenance treatment with prednisolone monotherapy and was controlled with methylprednisolone and cyclosporine A. Measurement of clinical parameters, including proteinuria, was important, and a renal biopsy finally established the diagnosis of IgG4-RKD. IgG4-RKD can present with progressive glomerular lesions and can be latent in cases diagnosed with diabetic kidney disease, particularly in patients with insulin insufficiency.
Keywords: Renal Insufficiency, Chronic, nephrotic syndrome, Immunoglobulin G4-Related Disease, Glomerulonephritis, Membranous, autoimmune pancreatitis, Humans, Male, Cyclosporine, Steroids, Methylprednisolone, Proteinuria, Acute Disease, Insulin
Background
Immunoglobulin G4-related disease (IgG4-RD) is a systemic fibroinflammatory disorder that can develop synchronously and metachronously in multiple organs. Commonly affected organs include the pancreas, salivary glands, lacrimal glands, bile ducts, retroperitoneum, and kidneys [1]. Type 1 autoimmune pancreatitis is an IgG4-RD that can trigger diabetes mellitus (DM), with reduced insulin production [2]. The prevalence of type 1 autoimmune pancreatitis in Japan increased from 2.2 per 100 000 persons in 2007 to 10.1 per 100 000 in 2016 [3,4]. Owing to an increased awareness of the disease and the development of guidelines, the number of patients, including those who have previously been overlooked or undiagnosed with IgG4-RD, is expected to increase in the future [4,5].
Renal tubular interstitial nephritis, the main pathological condition of IgG4-related kidney disease (IgG4-RKD), is sometimes complicated by glomerular disease [6]. Membranous nephropathy is the most common glomerulopathy, observed in 7% of IgG4-related tubular interstitial nephritis cases. Membranous nephropathy without tubular interstitial nephritis has also been reported [7,8]. However, the prevalence and clinical features of this disease remain unclear, and the clinical characteristics of patients with IgG4-related membranous nephropathy and concomitant DM remain unclear.
Diabetic nephropathy is a major complication of DM, and diabetic kidney disease (DKD) has been categorized to achieve a better prognosis in patients with DM [9]. Diabetic nephropathy typically develops from albuminuria 5 to 10 years after the onset of DM [10]. In contrast, diabetic nephropathy has been reported to develop within 5 years of onset [11] or to progress rapidly in a short period [12]. Subsequently, progression to overt proteinuria indicates a risk of renal dysfunction and end-stage renal failure [13]. However, atypical diabetes-related nephropathies without overt albuminuria [14,15], due to renin-angiotensin system inhibitors, nephrosclerosis, and interstitial fibrosis with tubular atrophy [14,16], should be considered to prevent renal regression. DKD is a chronic kidney disease encompassing classic diabetic and diabetes-related nephropathy with an atypical course.
Herein, we present a case of IgG4-RKD that developed into membranous nephropathy despite successful maintenance treatment with prednisolone for type 1 autoimmune pancreatitis. Physicians should be aware that IgG4-RKD with nephrotic syndrome can develop in patients with multiorgan IgG4-RD and DM.
Case Report
A 54-year-old Japanese man presented to our general hospital with symptoms of thirst, polydipsia, and polyuria. DM was diagnosed based on a high level of fasting plasma glucose (FPG) of 293 mg/dL, and glycated hemoglobin (HbA1c) of 16.3% (National Glycohemoglobin Standardization Program [NGSP]). He had been diagnosed with impaired glucose tolerance 4 years before the diagnosis of IgG4-RD. The patient presented with a diffusely enlarged pancreas, mainly the body and tail (Figure 1A); elevated serum IgG4 levels and multi-organ involvement; bile and pancreatic duct stenosis, demonstrated on endoscopic retrograde cholangiopancreatography (ERCP) and magnetic resonance cholangiopancreatography (MRCP) (Figure 1B); a soft tissue shadow in the para-aortic region observed on contrast-enhanced computed tomography (CT); and a positive response to glucocorticoid therapy. These clinical manifestations aligned with both the HISORt criteria [17] and the Japanese clinical diagnostic criteria for autoimmune pancreatitis [18]. Based on these congruent findings, type 1 autoimmune pancreatitis, sclerosing cholangitis, and retroperitoneal fibrosis were definitively diagnosed (Figure 1A–1C). Considering the diffuse enlargement of the pancreas, the decrease in endogenous insulin secretion (serum fasting C-peptide, 0.45 ng/mL), and the negative islet-related autoantibody test, pancreatic DM was diagnosed [2]. The patient was treated with oral glucocorticoids at an initial prednisolone dose of 25 mg/day. After tapering the oral prednisolone dose, the patient was treated with a maintenance dose of prednisolone 5 mg/day, without any recurrence of IgG4-RD manifestations, including cholangitis, retroperitoneal fibrosis, and autoimmune pancreatitis. The clinical course of this case is shown in Figure 2. Regarding DM, insulin secretion was insufficient owing to type 1 autoimmune pancreatitis and did not recover during the clinical course; however, plasma glucose levels were managed at an approximate HbA1c level of 6% using basal-bolus insulin therapy. One and a half years after initiating glucocorticoid treatment, the patient was admitted to our hospital with leg edema and overt proteinuria.
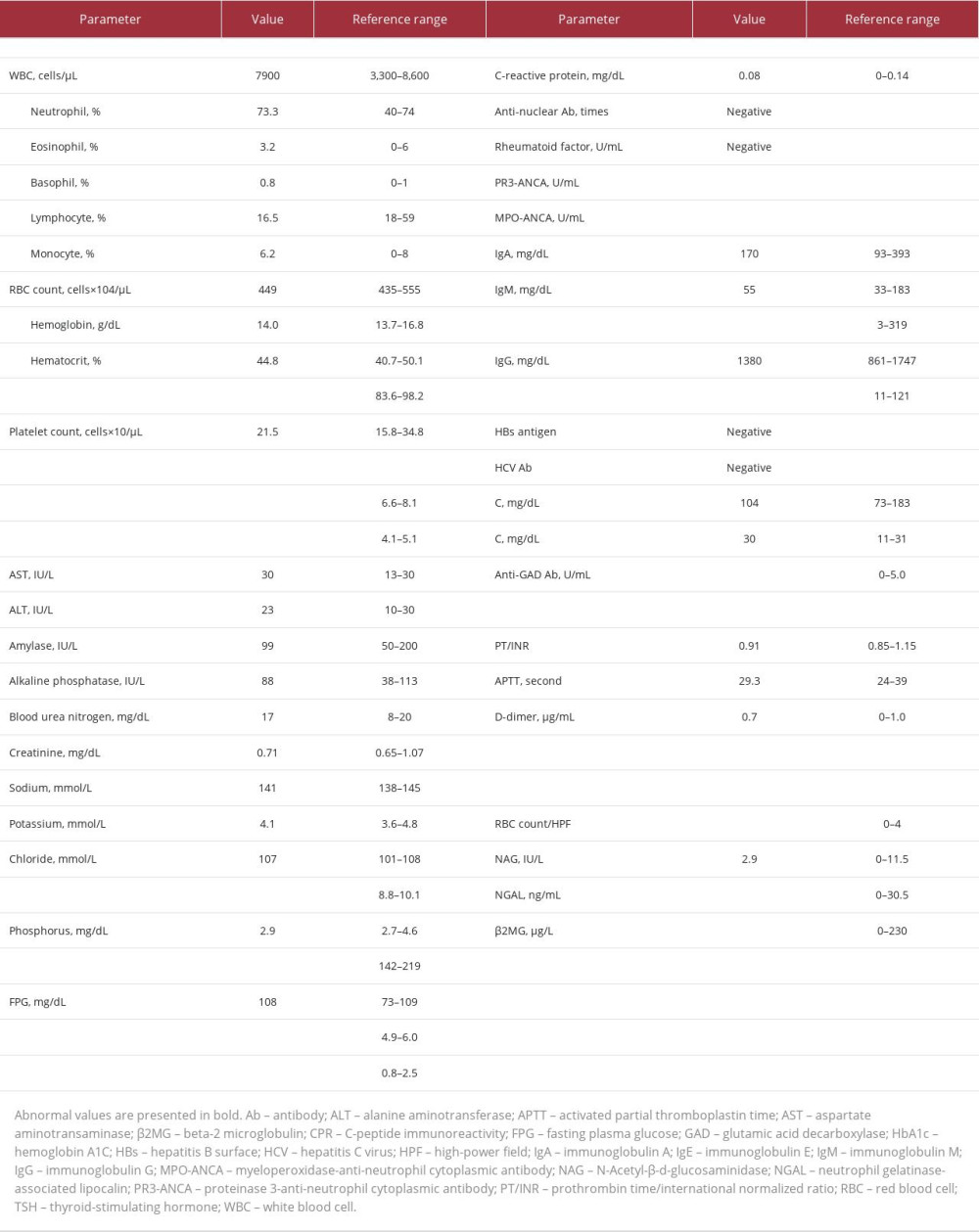
On physical examination, his consciousness was clear, and he had a height of 159.6 cm, a body weight of 53.5 kg, and a body mass index of 21.0 kg/m2. His blood pressure was 136/70 mm Hg, pulse rate was 81 beats per min, body temperature was 36.7 °C, oxygen saturation level was 96% (on room air), and he had bilateral lower leg pitting edema. Laboratory examinations revealed hypoproteinemia, hypoalbuminemia, and hyperlipidemia, although glucose metabolism was well managed (FPG, 108 mg/dL; HbA1c, 6.1% [NGSP]) [19] and the serum creatinine level was within the normal range of 0.71 mg/dL (Table 1). Additionally, urinalysis revealed severe proteinuria (4.3 g/day), without hematuria. CT revealed a diffusely atrophic pancreas, without renal abnormalities (Figure 1C, 1D). A renal biopsy was performed for further examination; however, no abnormalities were observed in the glomeruli or tubulointerstitium, using light microscopy (Figure 3A). Immunostaining revealed granular IgG deposits along the basement membrane (Figure 3B); however, it was negative for anti-phospholipase A2 receptor (PLA2R) antibody, which is the causative antigen of primary membranous nephropathy [7] (Figure 3C). Additionally, strong IgG4 staining was observed, although IgG1 deposition was also observed (Figure 3D). The serum anti-PLA2R antibody level was not measured in this case.
IgG4-related membranous nephropathy without tubular interstitial nephritis was diagnosed based on the history of IgG4-RD and renal pathology findings, and other secondary membranous nephropathies were ruled out. Glucocorticoids were changed from prednisolone (5 mg/day) to methylprednisolone because of the severe edema, and the dose was increased to 16 mg/day (Figure 2). However, despite an initial improvement in the proteinuria, we were unable to taper the patient’s glucocorticoids, owing to proteinuria flare-ups and nephrotic syndrome. Thus, the dose of methylprednisolone was increased to 24 mg/day, and cyclosporine A 100 mg/day was added. The 2-h post-dose level of cyclosporine A (C2), which indicates the therapeutic efficacy well [20], was measured during microemulsion cyclosporine A administration. It measured 813.3 ng/mL at 23 months, (2 weeks after 100 mg/day of cyclosporine A initiation) and 770.4 ng/mL at 29 months (6 months after 100 mg/day of cyclosporine A initiation) (Figure 2). The C2 levels had been maintained within the optimal range for idiopathic membranous nephropathy treatment of 600 ng/mL to 900 ng/mL [21]. Urinary protein and serum albumin levels decreased and increased, respectively, and serum IgG4 levels decreased markedly from 684 mg/dL to 141 mg/dL (reference range: 11–121 mg/dL) (Figure 2). Serum creatinine levels had been consistently within the reference range, and renal dysfunction had not been observed. He visited our hospital regularly and was managed with IgG4-RKD stable for 20 months.
Discussion
We have presented a case of IgG4-RKD complicated by pancreatic DM [22] manifesting as insulin insufficiency due to type 1 autoimmune pancreatitis. DKD is defined as a chronic kidney disease that develops and progresses in DM [23]. DKD includes various atypical diabetes-related renal diseases, with a decreased glomerular filtration rate without overt albuminuria and typical diabetic nephropathy [9]. IgG4-RKD presents with various findings ranging from acute or slow progression, such as tubulointerstitial nephritis or glomerular disease, membranous nephropathy, and immunoglobulin A nephropathy [7,24]; however, distinguishing the differential diagnosis from DKD is difficult. Additionally, although approximately 77% of cases of type 1 autoimmune pancreatitis are complicated with DM [25], the possibility of IgG4-RD should be considered. Thus, clinicians should rule out IgG4-RD when diagnosing DKD, especially in patients with decreased insulin secretion.
Nephrotic syndrome, a comorbidity in patients with DM, improved to stable remission after methylprednisolone and cyclosporine A treatment for IgG4-RKD. Membranous nephropathy manifests with proteinuria as a main clinical feature and presents characteristics of diffuse thickening and deposits on the epithelial side of the glomerular basement membrane [26]. Approximately 75% of adult membranous nephropathy cases have primary (idiopathic) origins, while secondary cases have a background of malignant disease, autoimmune disease, drugs, or infection [27]. PLA2R positivity is a key clue in the diagnosis of primary membranous nephropathy; thus, most cases can be differentiated based on the results of anti-PLA2R antibody staining [7]. In the present case, the diagnosis of IgG4-related membranous nephropathy was confirmed by renal pathology findings of granular deposition of IgG4 along the basement membrane and PLA2R negativity. Furthermore, no additional finding indicative of secondary membranous nephropathy was observed. Notably, the patient developed IgG4-related membranous nephropathy as a new lesion, even though he had been continuously receiving maintenance therapy with prednisolone 5.0 mg/day (maximum dose, 25 mg/day) for his systemic IgG4-RD (i.e., type 1 autoimmune pancreatitis, IgG4-related sclerosing cholangitis, and retroperitoneal fibrosis). Additionally, he presented with nephrotic syndrome, although IgG4-RKD primarily manifests as interstitial lesions, typically with decreased renal function without proteinuria or hematuria [6]. Thus, this case highlights that IgG4-RKD should even be considered in patients with massive proteinuria, including nephrotic syndrome, as a plausible combination of IgG4-RD and DM due to autoimmune pancreatitis.
Chronological and spatial multiplicity in the affected organs should be considered even after treatment for IgG4-RD. The lungs and lacrimal glands have a high incidence of remission induction failure; the therapeutic efficacy of remission depends on the affected organs [28]. In the present case, the membranous nephropathy went into remission with glucocorticoids and cyclosporine A; however, patients with membranous nephropathy refractory to treatment were also reported [7], although IgG4-related tubular interstitial nephritis responded to glucocorticoid therapy in 90% of cases [29]. Furthermore, the optimal treatment for each organ-specific IgG4-RD has not been established based on prospective randomized trials [4,30]. The present case suggests that strategies for organ-specific treatments along with general management are required for IgG4-RD. Clinicians should be aware that persistently high serum IgG4 levels after glucocorticoid therapy can be a predictive marker for IgG4-RD exacerbations [31]. Regular monitoring with evaluations for IgG4 levels and CT imaging, in the present case, confirmed that CT imaging might not capture new lesions and that an IgG4 level might be a good marker to detect them. Further, serum IgG4 levels have been reported as a potential marker of IgG4-RD recurrence [30,32]. This study suggests that systemic re-evaluations for IgG4-RD will be required when IgG4 levels show re-elevation, even in the remission of the known IgG4-RD [33]. In contrast, elevated serum IgG4 levels are not specific for IgG4-RD [1], and histology was essential for confirming the diagnosis of IgG4-RKD. When monitoring the disease, measurements of organ functions (including creatinine, proteinuria, bilirubin, elastase, and HbA1c levels) were crucial to detect organ damage, such as membranous nephropathy. Further clinical decisions, including treatment changes, should be made on this basis and not solely based on serum IgG4 levels.
Underlying pathogenetic differences between IgG4-RD and IgG4-related membranous nephropathy may be revealed by the response to glucocorticoid monotherapy and combination therapy with glucocorticoids and immunosuppressants. IgG4-RKD with nephrotic syndrome occurred during low-dose (5–7.5 mg/day) prednisolone monotherapy, even though other IgG4-RDs maintained remissions and were controlled with a combination of methylprednisolone and cyclosporine A. IgG4-RD relapse occurs in approximately 24% to 54% of patients when they are treated with glucocorticoid monotherapy [34]. In Japan, glucocorticoid monotherapy is the standard treatment for type 1 autoimmune pancreatitis; however, a high relapse rate, 30.3% under maintenance therapy, has been noted [32]. Thus, low-dose glucocorticoid treatment manages to prevent relapses of autoimmune pancreatitis [32]. However, in prospective studies [28,35], combination therapy with glucocorticoids and immunosuppressive agents proved more effective for IgG4-RD than did glucocorticoid monotherapy for remission and the prevention of relapse. This case report may highlight the effectiveness of combination therapy with glucocorticoids and cyclosporine A for IgG4-RD, including IgG4-related membranous nephropathy. In this context, IgG4-RD may have various pathogeneses [7] that lead to different responses to glucocorticoids and immunosuppressants among affected organs, although it is a systemic disease.
This study had two limitations. First, although IgG4-related membranous nephropathy was stable in remission for 20 months after initiation of glucocorticoids and immunosuppressants, further observation is required to confirm the long-term prognosis. Second, further comparative studies with case series are required to optimize the IgG4-RD and IgG4-RKD treatments.
Conclusions
We have reported a case of IgG4-RKD with membranous nephropathy during the treatment of autoimmune pancreatitis-associated DM. This study highlights that clinicians should be aware of the occurrence of IgG4-RKD, along with DKD, when they encounter patients with proteinuria and insulin insufficiency. IgG4-RKD with membranous nephropathy and nephrotic syndrome is a rare but important finding in patients with multiorgan IgG4-RD and can occur despite treatment with prednisolone. Renal biopsy with immunostaining is key to diagnosing IgG4-RKD. Methylprednisolone and cyclosporine A can maintain the remission of IgG4-related membranous nephropathy, even in patients with a high risk for disease relapse, including those with multiorgan IgG4-RD.
Figures
References:
1.. Kamisawa T, Zen Y, Pillai S, Stone JH, IgG4-related disease: Lancet, 2015; 385; 1460-71
2.. Shiokawa M, Kodama Y, Kuriyama K, Pathogenicity of IgG in patients with IgG4-related disease: Gut, 2016; 65; 1322-32
3.. Masamune A, Kikuta K, Hamada S, Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016: J Gastroenterol, 2020; 55; 462-70
4.. Chen LYC, Mattman A, Seidman MA, Carruthers MN, IgG4-related disease: What a hematologist needs to know: Haematologica, 2019; 104; 444-55
5.. Blaho M, Dítě P, Kunovský L, Kianička B, Autoimmune pancreatitis – an ongoing challenge: Adv Med Sci, 2020; 65; 403-8
6.. Kawano M, Saeki T, Nakashima H, IgG4-related kidney disease and retroperitoneal fibrosis: An update: Mod Rheumatol, 2019; 29; 231-39
7.. Alexander MP, Larsen CP, Gibson IW, Membranous glomerulonephritis is a manifestation of IgG4-related disease: Kidney Int, 2013; 83; 455-62
8.. Cravedi P, Abbate M, Gagliardini E, Membranous nephropathy associated with IgG4-related disease: Am J Kidney Dis, 2011; 58; 272-75
9.. Tuttle KR, Bakris GL, Bilous RW, Diabetic kidney disease: A report from an ADA Consensus Conference: Diabetes Care, 2014; 37; 2864-83
10.. Gheith O, Farouk N, Nampoory N, Diabetic kidney disease: World wide difference of prevalence and risk factors: J Nephropharmacol, 2015; 5; 49-56
11.. Stephenson JM, Fuller JH, Microalbuminuria is not rare before 5 years of IDDM. EURODIAB IDDM Complications Study Group and the WHO Multinational Study of Vascular Disease in Diabetes Study Group.: J Diabetes Complications, 1994; 8; 166-73
12.. Morino J, Hirai K, Kaneko S, Two cases of advanced stage rapidly progressive diabetic nephropathy effectively treated with combination therapy including RAS blocker, GLP-1 receptor agonist and SGLT-2 inhibitor: CEN Case Rep, 2019; 8; 128-33
13.. Yokoyama H, Sone H, Oishi M, Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15): Nephrol Dial Transplant, 2009; 24; 1212-19
14.. Afkarian M, Zelnick LR, Hall YN, Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014: JAMA, 2016; 316; 602-10
15.. Kume S, Araki S, Ugi S, Secular changes in clinical manifestations of kidney disease among Japanese adults with type 2 diabetes from 1996 to 2014: J Diabetes Investig, 2019; 10; 1032-40
16.. Shimizu M, Furuichi K, Toyama T, Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy: Diabetes Care, 2013; 36; 3655-62
17.. Chari ST, Smyrk TC, Levy MJ, Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience: Clin Gastroenterol Hepatol, 2006; 4; 1010-16
18.. Kawa S, Kamisawa T, Notohara K, Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018: Revision of Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2011: Pancreas, 2020; 49; e13-e14
19.. Laiteerapong N, Ham SA, Gao Y, The legacy effect in type 2 diabetes: Impact of early glycemic control on future complications (the diabetes & aging study): Diabetes Care, 2019; 42; 416-26
20.. Nashan B, Bock A, Bosmans JL, Use of neoral C monitoring: a European consensus: Transpl Int, 2005; 18; 768-78
21.. Saito T, Iwano M, Matsumoto K, Significance of combined cyclosporine-prednisolone therapy and cyclosporine blood concentration monitoring for idiopathic membranous nephropathy with steroid-resistant nephrotic syndrome: A randomized controlled multicenter trial: Clin Exp Nephrol, 2014; 18; 784-94
22.. ElSayed NA, Aleppo G, Aroda VR, 2. Classification and diagnosis of diabetes: Standards of care in diabetes-2023.: Diabetes Care, 2023; 46(Suppl. 1); S19-S40
23.. Yamazaki T, Mimura I, Tanaka T, Nangaku M, Treatment of diabetic kidney disease: Current and future.: Diabetes Metab J, 2021; 45; 11-26
24.. Wada Y, Saeki T, Yoshita K, Development of IgG4-related disease in a patient diagnosed with idiopathic membranous nephropathy: Clin Kidney J, 2013; 6; 486-90
25.. Okazaki K, Kawa S, Kamisawa T, Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, 2013 I. Concept and diagnosis of autoimmune pancreatitis.: J Gastroenterol, 2014; 49; 567-88
26.. Alsharharhan L, Beck LH, Membranous nephropathy: Core Curriculum 2021: Am J Kidney Dis, 2021; 77; 440-53
27.. Gu Y, Xu H, Tang D, Mechanisms of primary membranous nephropathy: Biomolecules, 2021; 11; 513
28.. Wang L, Zhang P, Wang M, Failure of remission induction by glucocorticoids alone or in combination with immunosuppressive agents in IgG4-related disease: A prospective study of 215 patients: Arthritis Res Ther, 2018; 20; 65
29.. Saeki T, Nishi S, Imai N, Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis: Kidney Int, 2010; 78; 1016-23
30.. Carruthers MN, Stone JH, Deshpande V, Khosroshahi A, Development of an IgG4-RD Responder Index: Int J Rheumatol, 2012; 2012; 259408
31.. Suzuki D, Shimizu K, Tokushige K, Relative rise of serum IgG4 levels after steroid therapy for autoimmune pancreatitis predicts the likelihood of relapse: Pancreas, 2018; 47; 412-17
32.. Kubota K, Kamisawa T, Okazaki K, Low-dose maintenance steroid treatment could reduce the relapse rate in patients with type 1 autoimmune pancreatitis: A long-term Japanese multicenter analysis of 510 patients: J Gastroenterol, 2017; 52; 955-64
33.. Peng Y, Li JQ, Zhang PP, Clinical outcomes and predictive relapse factors of IgG4-related disease following treatment: A long-term cohort study: J Intern Med, 2019; 286; 542-52
34.. Zongfei J, Lingli C, Ying S, Clinical and pathological predictors of relapse in IgG4-related disease: Arthritis Res Ther, 2022; 24; 106
35.. Yunyun F, Yu C, Panpan Z, Efficacy of Cyclophosphamide treatment for immunoglobulin G4-related disease with addition of glucocorticoids: Sci Rep, 2017; 7; 6195
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250