16 October 2023: Articles 
Bilirubin Elevation Caused by Naproxen Overdose: A Case Report Highlighting Laboratory Interference
Unusual clinical course, Unexpected drug reaction
Olivia Heutlinger1DEF*, Tobin Mathew1DEF, Dylann FujimotoDOI: 10.12659/AJCR.941267
Am J Case Rep 2023; 24:e941267
Abstract
BACKGROUND: Overdoses on over-the-counter (OTC) drugs are increasing in the United States, which includes widely available non-steroidal anti-inflammatory drugs (NSAIDs) like naproxen. Symptoms of NSAID toxicity are well known and nonspecific, including nausea, vomiting, abdominal pain, and headaches. Extreme cases can present with confusion, seizures, and renal failure.
CASE REPORT: We present the case of 63-year-old man with a history of hyperthyroidism and polysubstance use who had an elevated total bilirubin level after attempting suicide via ingestion of 16 tablets of naproxen. The patient presented with vague abdominal pain and nausea in the setting of 2 weeks of worsening psychiatric symptoms, including suicidal ideation. Vital signs, physical examination, and review of systems revealed no significant findings. Medical workup was notable only for an elevated total bilirubin level; workup for hemolysis, biliary stasis, hepatic dysfunction was all within normal limits. Direct bilirubin was not elevated. The patient received intravenous fluids and antiemetic medications, and indirect hyperbilirubinemia resolved by the following day. After ruling out other causes of hyperbilirubinemia, it was determined that his elevated bilirubin was due a naproxen metabolite, O-desmethylnaproxen (ODMN), that has been shown to interfere with certain bilirubin assays when naproxen is ingested over the therapeutic dose.
CONCLUSIONS: Supratherapeutic naproxen ingestion can lead to laboratory findings of elevated total bilirubin in some assays due to ODMN interference. With the rise in suicide attempts in the United States with OTCs, clinicians should consider laboratory error in such clinical circumstances where the clinical data does not fit the history and physical examination.
Keywords: desmethylnaproxen, Drug Overdose, Hyperbilirubinemia, naproxen, Male, Humans, United States, Middle Aged, bilirubin, Anti-Inflammatory Agents, Non-Steroidal, Nausea, Nonprescription Drugs, Abdominal Pain
Background
Naproxen is a non-steroidal anti-inflammatory drug (NSAID) commonly used for its anti-inflammatory and analgesic effects. It is part of the 2-arylpropionic acid class of NSAIDs and is completely absorbed when taken orally. It has a 95% bio-availability, binding avidly to albumin at doses up to 500 mg, after which the fraction of unbound naproxen increases. It is metabolized to O-desmethylnaproxen (ODMN) and then is glucuroconjugated prior to elimination [1]. The half-life of naproxen varies by patient age and renal function, and over 80% is eliminated in the urine.
Naproxen inhibits the cyclooxygenase (COX) enzymes, COX-1 and COX-2. COX-1 is constitutively active in the gastrointestinal tract, and COX-2 exhibits peak expression at sites of inflammation. COX enzymes are responsible for the production of pros-taglandins, thromboxane, and levuloglandins. Prostaglandins are molecules that mediate pain perception and inflammation. COX-2 is also expressed in parts of the central nervous system and likely plays a role in nociception [2]. As such, inhibition of the COX enzymes and prostaglandin synthesis helps in alleviating pain related to inflammation.
Naproxen is readily available as an over-the-counter (OTC) medication, making overdose more common given its easy accessibility. Toxic ingestion of drugs can often lead to a variety of clinical signs and symptoms, which typically follow a known pattern. Naproxen toxicity is similar to that of other NSAID toxicity, which includes nausea and vomiting, abdominal pain, headaches, and drowsiness, with more severe cases presenting with seizures, metabolic acidosis, renal failure, and even coagulopathy [3,4]. The following case presents a patient who arrived at the hospital following attempted suicide via naproxen overdose and was found to have an isolated elevated bilirubin level without an otherwise apparent organic explanation.
Case Report
A 63-year-old male patient presented to the Emergency Department with nonspecific left-sided abdominal pain and nausea without emesis after attempting suicide via ingestion of 16 tablets of 500 mg naproxen. The patient reported 2 weeks of worsening depression, increased auditory hallucinations, with suicidal and homicidal content, and paranoid delusions. These symptoms worsened in the 3 days prior to presentation. Of note, the patient had been smoking meth-amphetamine and marijuana daily and drinking a half pint of vodka daily for multiple weeks leading up to hospitalization. The patient denied chest pain, dyspnea, visual disturbances, dizziness, lightheadedness, visual hallucinations, confusion, ataxia, and any genitourinary lesions or symptoms. He had no prior history of jaundice, or indirect hyperbilirubinemia on laboratory test results. The patient was afebrile, with a temperature of 36.5°C (97.7°F). Vital signs were all within normal limits, with blood pressure 129/74 mmHg, heart rate 74 beats/ min, respiratory rate 14 breaths/min, and oxygen saturation of 100% on ambient air. Physical examination was unremarkable, with the absence of abdominal tenderness or distention, jaundice, and scleral or sublingual icterus. He had a medical history of hyperthyroidism, which was previously treated with methimazole and propranolol but the patient was not currently on thyroid replacement therapy, as well as polysubstance use disorder, including the use of methamphetamine, fentanyl, alcohol, and marijuana.
In the Emergency Department, the patient received 1 L of normal saline and 4 mg of ondansetron. Laboratory findings were significant for an elevated total bilirubin level to 5.7 mg/dL, with direct bilirubin of 0.1 mg/dL. His baseline total bilirubin was 0.6 mg/dL, from an admission 2 months prior. Additional laboratory testing results can be seen in Table 1. A urine drug screen was positive for amphetamines and marijuana, and serum ethanol was negative. He was admitted to the medical ward for further workup of hyperbilirubinemia. Further investigation revealed an ingested naproxen level of 92.4 mg/kg. In addition to those results listed in Table 1, the patient also had a negative peripheral smear, with no abnormal red blood cell morphology (eg, schistocytes) and a urinalysis that was positive for moderate bilirubin, without urobilinogen. A right-upper quadrant ultrasound did not demonstrate biliary ductal dilation, signs of cholecystitis, or hepatosplenomegaly. The patient was placed on a psychiatric hold and received isotonic fluids and ondansetron as needed for nausea. His abdominal pain resolved overnight without gastrointestinal decontamination, and the patient’s total bilirubin level was normal at 0.9 mg/dL the next morning. The patient was transferred to The Inpatient Psychiatric Unit for further management and treatment.
Discussion
This case demonstrates spurious indirect hyperbilirubinemia caused by naproxen toxicity. This conclusion was established once the other causes of hyperbilirubinemia, such as hemolysis, hepatic dysfunction, biliary obstruction, and genetic causes, were investigated and found negative. Other causes, including Wilson Disease, biliary extravasation, shunt hyperbilirubinemia, portosystemic shunt, and hyperthyroidism, are detailed in Table 2. However, the patient’s indirect hyperbilirubinemia normalized within 24 h without intervention. This is likely the result of the inference of a naproxen metabolite, ODMN, with a particular method used by many bilirubin assays to assess for serum bilirubin levels.
Bilirubin levels are commonly obtained in clinical practice and can be elevated in a variety of disease states. Unconjugated (indirect) bilirubin is produced as heme and is broken down from red blood cells. Conjugated (direct) bilirubin is produced from unconjugated bilirubin using the enzyme UDP-glucanosyltransferase in hepatocytes. Our patient had an elevated total bilirubin level without direct hyperbilirubinemia, indicating an elevated indirect bilirubin. Increased breakdown of hemoglobin from hemolysis can cause indirect hyperbilirubinemia; however, our patients’ normal lactate dehydrogenase (163 mU/mL) and haptoglobin (201 mg/dL) ruled out hemolysis. Intrahepatic biliary obstruction can also lead to an indirect hyperbilirubinemia if there is enough obstruction and damage to hepatocytes. Our patients’ right-upper-quadrant ultrasound showed no biliary ductal dilation, signs of cholecystitis, or hepatosplenomegaly, suggesting biliary obstruction was not present. Chronic hepatitis and advanced cirrhosis can lead to indirect hyperbilirubinemia through reduced hepatic uptake of indirect bilirubin from the serum. Genetic abnormalities, such as Gilbert syndrome or Crigler-Najjar syndrome, can affect the bilirubin conjugation pathway [5]. There was no sign of liver disease on the right-upper-quadrant ultrasound, and the liver panel results were within normal limits (aspartate transaminase 24 IU/L; alanine transaminase, 15 IU/L; alkaline phosphatase, 82 IU/L). In both Gilbert syndrome and Crigler-Najjar syndrome there is a defect in the UDP-glucanosyltransferase enzyme that causes elevations in indirect bilirubin. Gilbert syndrome was considered in this patient, as it typically presents with mild elevations in unconjugated bilirubin that are largely asymptomatic, particularly in states of stress. Crigler-Najjar syndrome is more severe and is typically diagnosed in patients within their first year of life [6]. This patient had normal bilirubin levels during previous admissions and states of stress, which makes either syndrome less likely. Additionally, the bilirubin normalized within 1 day, which would be less common in an enzymatic deficiency. This patient’s nausea was treated with ondansetron, a 5HT-3 serotonin-receptor antagonist metabolized primarily in the liver, which can cause transient elevations in liver function tests [7]. However, ondansetron typically causes a hepatocellular pattern of injury, so it would be an unlikely cause of an indirect hyperbilirubinemia. Additionally, the use of serotoninergic medications can lead to serotonin syndrome in patients taking other serotonergic medications. However, our patient’s symptoms improved without further signs of clinical decompensation.
Naproxen is part of the propionic acid class of NSAIDs and is structurally and physiologically unrelated to bilirubin (Figure 1). Naproxen is metabolized to ODMN, and both naproxen and its metabolite are excreted as conjugates, primarily in the urine [8–10]. Naproxen has a mean half-life of 13 h, while ODMN has a half-life of 1 h [10,11]. Naproxen overdoses are usually mild, and severe toxicity is rare [4]. There is no specific toxicity cutoff for naproxen, although it is generally accepted severe toxicity occurs at levels over 400 mg/kg and symptoms are unlikely at less than 100 mg/kg, like with other NSAIDs in the propionic acid group (ie, ibuprofen) [12].
Our patient reported abdominal pain in the setting of ingestion of 92.4 mg/kg of naproxen, which is significantly under the 400 mg/kg level for severe toxicity. There is no standard treatment for naproxen overdose, as the role for anti-toxins such as activated charcoal is currently unclear, and hemodialysis would be ineffective in clearance of ODMN given naproxen’s high binding to albumin in serum [4]. It is possible there was biliary stasis in this patient in the setting of elevated serum naproxen; however, there was no accompanying laboratory or imaging findings suggestive of biliary stasis, as previously mentioned. Additionally, hemolysis was effectively ruled out with laboratory testing, as discussed. The patient may have had underlying Gilbert syndrome, exacerbated by the stress of an active overdose and suicide attempt, but previous admissions have never led to elevated bilirubin levels. In the absence of typical organic causes of hyperbilirubinemia, the possibility of laboratory error was considered.
Supratherapeutic serum naproxen ingestion has been shown to affect certain laboratory assays [8,13]. Evidence suggests that ODMN interacts with total bilirubin assays that use the time-endpoint diazo-based Jendrassic and Grof method, which uses the reaction between bilirubin and diazotized sulfanilic acid to measure total serum bilirubin levels [8,13]. ODMN does not interfere with direct bilirubin assays obtained using the same method. This pattern of interference has been shown with several chemistry analyzers that use the Jendrassic and Grof method, including the Beckman Synchron DxC800 assay and others, including the Siemens Dimension Vista assays [8,13]. Of note, therapeutic doses of naproxen have not been shown to interfere with any bilirubin assays. The hospital running the laboratory testing for this patient used the Beckman Synchron DxC800 timed-endpoint diazo method for total bilirubin, which is known to have interference from both naproxen and ODMN at elevated serum levels of naproxen. The Jendrassic and Grof method used by the Beckman Synchron DxC800 assay measures direct and total bilirubin through their reaction with diazotized sulfanilic acid in the presence of caffeine. While there have been multiple hypotheses as to why ODMN only interacts with unconjugated bilirubin levels, studies performed suggest that acidic pH of conjugated bilirubin reagents inhibits the reaction of ODMN with diazo salts, leading to no interference between the compound and reported direct bilirubin levels [13]. The patient’s return to normal total bilirubin levels within 24 h of naproxen ingestion with no further abnormalities is suggestive that this interference was the cause of this laboratory finding.
Of note, the Beckman Synchron AU680 assay does not exhibit this same pattern of interference with ODMN in the setting of elevated serum naproxen levels. The AU680 assay measures total and direct bilirubin using a stable diazo salt in the presence of surfactant and caffeine. The primary difference between this and the DxC800 assay is the endpoint absorbance, with the AU680 assay measuring endpoint absorbance at 570 nm, and the DxC800 assay at 520 nm [13]. In the AU680 assay, diazo-reacted ODMN exhibits peak absorbance between 400 nm and 540 nm, versus the DxC method, in which peak absorbance occurs between 450 and 560 nm. The AU680 assay endpoint absorbance is beyond the range in which a change in diazo-reacted ODMN absorbance is observed, unlike the DxC800 assay, in which the endpoint absorbance is close to the peak absorbance of the ODMN-diazo reaction. As such, the AU650 assay is unlikely to produce any laboratory value anomaly in the setting of naproxen overdose.
The overall rates of self-poisoning suicide attempts, specifically via overdose on OTC analgesics like naproxen, have increased by 33.5% over the past 2 decades in the United States [14]. According to the Center for Disease Control, self-poisoning was the third leading cause of successful suicide attempts in all age groups from 2000 to 2020 [15]. Self-poisoning accounted for 16% of successful attempts, behind firearms (51.4%) and suffocation (24.7%) (WISQARS) [15]. Of all drugs available in OTC formulations, analgesics and antihistamines are the most frequently used in suicide attempts and completions across all age groups [16]. In the case of overdose using NSAIDs, it is important to monitor renal, gastrointestinal, hepatic, hematologic, neurologic, and biochemical function for various organ toxicities and laboratory abnormalities [17]. With the rise of suicide attempts using NSAIDs like naproxen, clinicians will likely see increasing frequencies of such attempts in the hospital, with the potential of laboratory abnormalities such as bili-rubin, as discussed in this case. It is important for clinicians to be able to recognize such anomalies and consider interference as confounders of data in clinical decision making. However, these should remain diagnoses of exclusion.
Conclusions
In the setting of naproxen overdose, the patient’s laboratory workup can demonstrate an unexplained elevated total bili-rubin level due to the interference of ODMN with certain total bilirubin assays. As observed in this patient, it is important to investigate all potential causes of indirect hyperbilirubinemia and have a broad differential when evaluating patients presenting in the Emergency Department. When laboratory abnormalities are present with no identifiable underlying cause and resolve without specific intervention, it is important to consider laboratory interference as the potential cause.
References:
1.. Davies NM, Anderson KE, Clinical pharmacokinetics of naproxen: Clin Pharmacokinet, 1997; 32(4); 268-93
2.. Leung GJ, Rainsford KD, Kean WF, Osteoarthritis of the hand II: Chemistry, pharmacokinetics and pharmacodynamics of naproxen, and clinical outcome studies: J Pharm Pharmacol, 2014; 66(3); 347-57
3.. Lelièvre B, Drouillard I, Thill C, Severe poisoning with naproxen causing coagulopathy: Basic Clin Pharmacol Toxicol, 2020; 126(5); 458-63
4.. Brutzkus JS, M, Varacallo M: Naproxen, 2023, Treasure Island, FL, StatPealrs Publishing [cited 2023, Jan]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK525965/?report=classic
5.. Kraemer D, Scheurlen M, [Gilbert disease and type I and II Crigler-Najjar syndrome due to mutations in the same UGT1A1 gene locus.]: Med Klin (Munich), 2002; 97(9); 528-32 [in German]
6.. Fabris L, Cadamuro M, Okolicsanyi L, The patient presenting with isolated hyperbilirubinemia: Dig Liver Dis, 2009; 41(6); 375-81
7.. Griddine A, Bush JS, Ondansetron: StatPearls [Internet] Feb 15, 2023, Treasure Island (FL), StatPearls Publishing
8.. Dasgupta A, Langman LJ, Johnson M, Chow L, Naproxen metabolites interfere with certain bilirubin assays: Elimination of interference by using a Roche bilirubin assay on the Hitachi 917 analyzer: Am J Clin Pathol, 2010; 133(6); 878-83
9.. : LiverTox: Clinical and research information on drug-induced liver injury [Internet], 2012, Bethesda (MD), National Institute of Diabetes and Digestive and Kidney Diseases -. Naproxen. [Updated 2020 Mar 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548159/
10.. Segre EJ, Naproxen metabolism in man: J Clin Pharmacol, 1975; 15(4 Pt. 2); 316-23
11.. Vree TB, Van Den Biggelaar-Martea M, Verwey-Van Wissen CP, The pharmacokinetics of naproxen, its metabolite O-desmethylnaproxen, and their acyl glucuronides in humans. Effect of cimetidine: Br J Clin Pharmacol, 1993; 35(5); 467-72
12.. Smolinske SC, Hall AH, Vandenberg SA, Toxic effects of nonsteroidal anti-inflammatory drugs in overdose. An overview of recent evidence on clinical effects and dose-response relationships: Drug Saf, 1990; 5(4); 252-74
13.. Saifee NH, Ranjitkar P, Greene DN, Factors influencing naproxen metabolite interference in total bilirubin assays: Clin Biochem, 2016; 49(6); 514-17
14.. Hopkins AG, Spiller HA, Kistamgari S, Suicide-related over-the-counter analgesic exposures reported to United States poison control centers, 2000–2018: Pharmacoepidemiol Drug Saf, 2020; 29(9); 1011-21
15.. , Web-based Injury Statistics Query and Reporting System (WISQARS). [online], 2022 Available from: www.cdc.gov/injury/wisqars
16.. Shoib S, Patel V, Khan S, Over-the-counter drug use in suicidal/self-harm behavior: Scoping review: Health Sci Rep, 2022; 5(3); e662
17.. Hunter LJ, Wood DM, Dargan PI, The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose: Open Access Emerg Med, 2011; 3; 39-48
18.. Singh A KT, Jialal I: Unconjugated hyperbilirubinemia, 2023, StatPearls
Tables
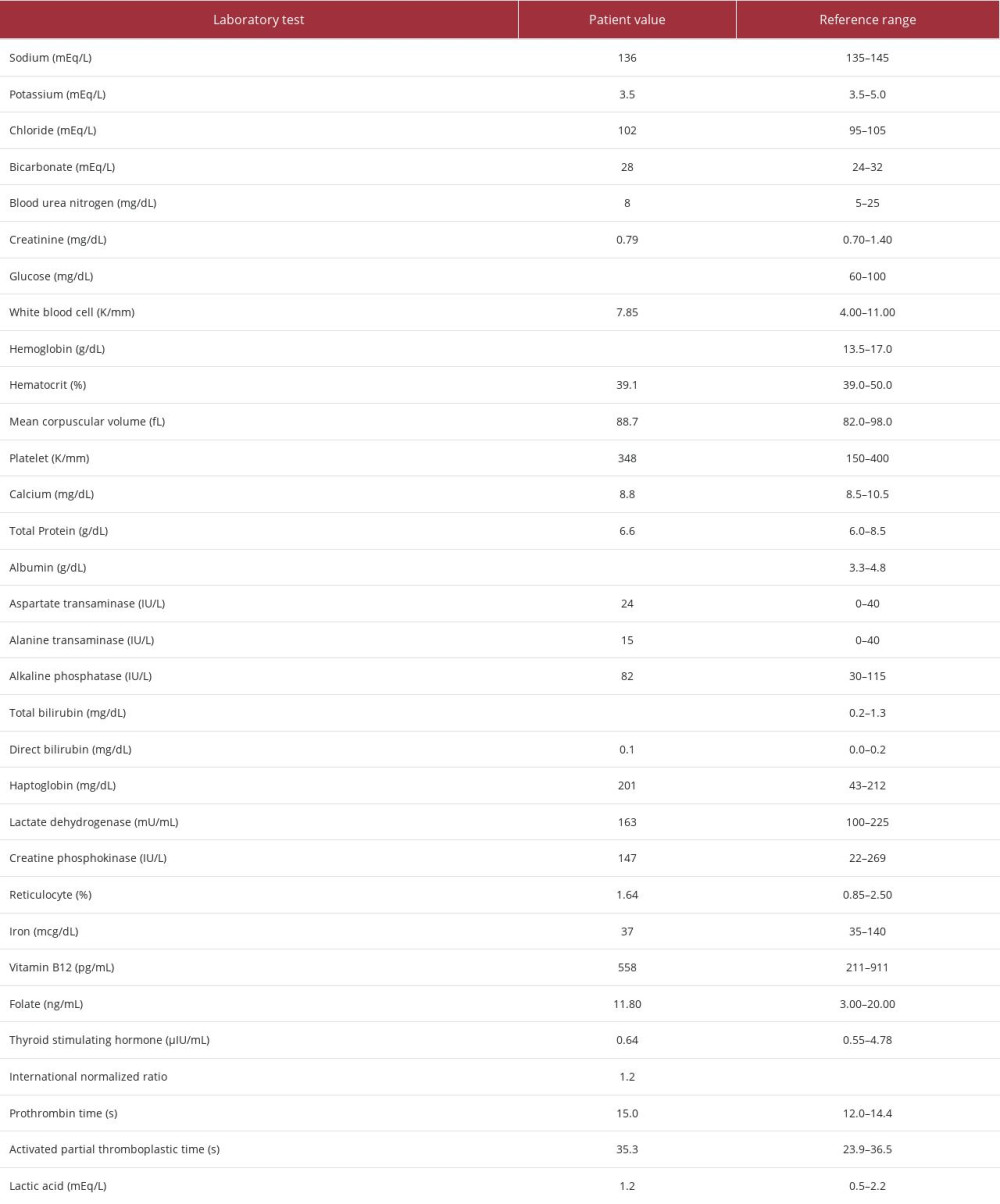 Table 1.. Laboratory findings of the patient on admission. Bolded values represent the abnormal findings.
Table 1.. Laboratory findings of the patient on admission. Bolded values represent the abnormal findings.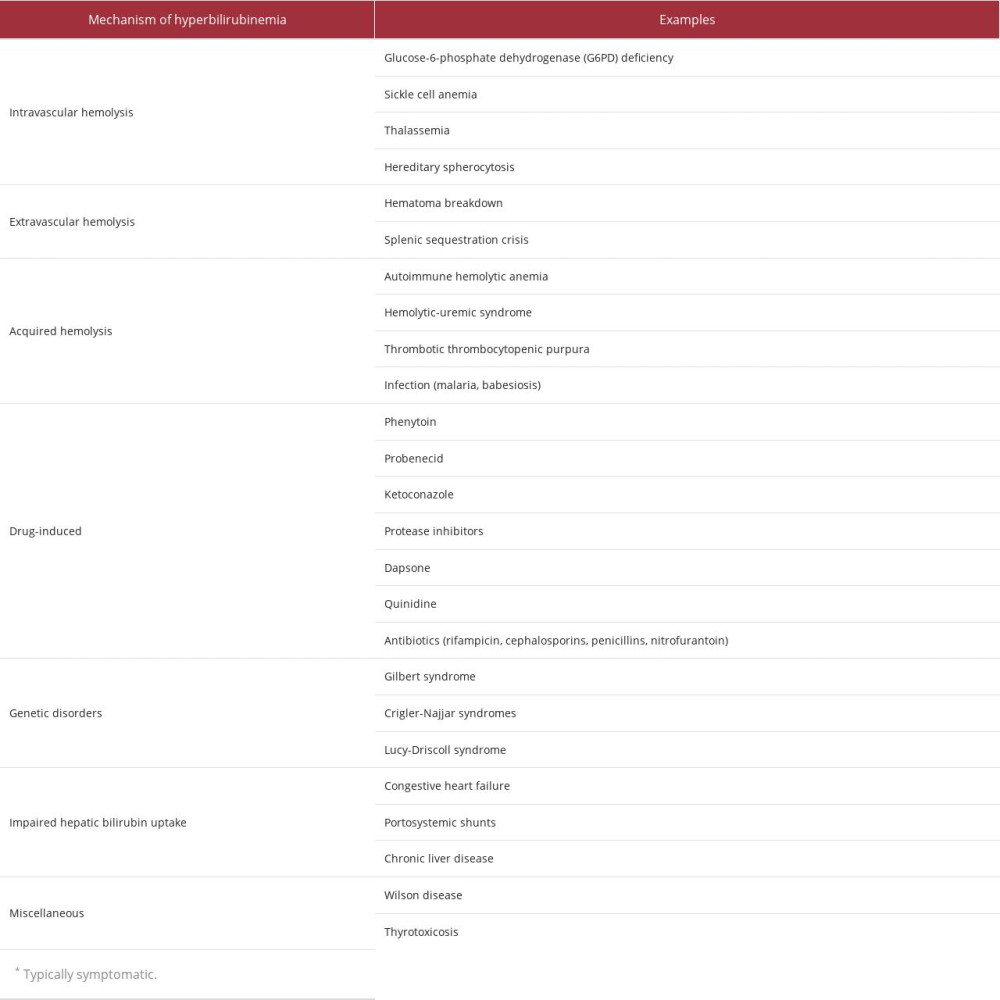 Table 2.. Differential diagnosis of indirect hyperbilirubinemia [18].
Table 2.. Differential diagnosis of indirect hyperbilirubinemia [18]. Table 1.. Laboratory findings of the patient on admission. Bolded values represent the abnormal findings.
Table 1.. Laboratory findings of the patient on admission. Bolded values represent the abnormal findings. Table 2.. Differential diagnosis of indirect hyperbilirubinemia [18].
Table 2.. Differential diagnosis of indirect hyperbilirubinemia [18]. In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








