07 December 2023: Articles 
Immune-Mediated Non-Infectious Periaortitis After Aortic Graft Replacement Surgery: A Case Report Highlighting the Need for Immunosuppressive Therapy
Unusual clinical course, Challenging differential diagnosis, Unusual setting of medical care
Yuki Hiramatsu1ABCDEF, Eiji Hiraoka1ABCDEF*, Hikaru Ito1ABCDEF, Keiichi Iwanami2ABCDEF, Joji Ito3ABCDEF, Minoru Tabata34ABCDEFDOI: 10.12659/AJCR.941428
Am J Case Rep 2023; 24:e941428
Abstract
BACKGROUND: A non-infectious inflammatory reaction against replaced aortic graft for aortic dissection often manifests as fever, malaise, and peri-graft effusion. It usually lasts less than 1 month and subsides spontaneously without immunosuppressive treatment.
CASE REPORT: A 49-year-old man underwent ascending aorta and total arch replacement for acute thoracic aortic dissection. He had fever, malaise, nausea, and elevated serum C-reactive protein for 1 month postoperatively. Pathological examination of the aorta revealed no aortitis, and repeated blood cultures were negative. We also noted periaortic graft fluid collection, and a small amount of pleural and pericardial effusions. We suspected post-pericardiotomy syndrome. Colchicine and prednisolone were administered, with an excellent clinical response. Three weeks after discontinuation of a 7-week prednisolone treatment, the same symptoms recurred and gradually worsened. Prednisolone was restarted 6 months after the first surgery, with good clinical response. Thereafter, he developed left-sided weakness and dysarthria, being diagnosed as ischemic stroke. Contrast-enhanced computed tomography revealed fluid collection with contrast leak around the aortic grafts, suggesting peel dehiscence, and thrombus formation in anastomotic pseudoaneurysm. He underwent surgical repair. He was diagnosed with non-infectious periaortitis, likely due to an immune reaction to the grafts, based on an excellent clinical response to immunosuppressive therapy.
CONCLUSIONS: We report a case of non-infectious periaortitis around a thoracic aortic graft, probably with an immune-mediated mechanism, requiring immunosuppressive treatment. When fever persists after aortic graft replacement surgery, non-infectious periaortitis should be considered and immunosuppressive treatment should be considered to prevent critical complications of anastomotic pseudoaneurysm and graft dehiscence.
Keywords: Aneurysm, Dissecting, Aorta, Thoracic, Immunity, Active, Immunosuppressive Agents
Background
Currently used aortic grafts consist of knitted or woven Dacron with bovine collagen or gelatin impregnated in its inner layer for the prevention of blood permeability [1]. Aortic graft replacement is often associated with an inflammatory response, such as fever, elevated C-reactive protein (CRP), and elevated white blood cell (WBC) count [2–4]. One of the mechanisms of inflammation may be a response against impregnated collagen and gelatin [2–4]. They are usually degraded and absorbed within 1 month, coinciding with the waning of the inflammatory reaction [1,5]. Another possible cause of the inflammation is endotoxin as a contaminant in bovine collagen- and gelatin-coated grafts [5]. Newer generation of grafts, Triplex™ [Vascutek, Terumo, Renfrewshire, United Kingdom (UK)], are three-layered uncoated devices. It does not include these animal-derived constituents, so theoretically it is expected to have lower risk of inflammatory reactions [6].
These inflammatory reactions usually subside spontaneously within 1–2 weeks after graft replacement surgery, seldom extending beyond 1 month [2–4,7]. We report herein a patient who developed fever, fatigue, and elevated CRP, which persisted as long as 6 months, which required treatment with prednisolone and methotrexate, suggesting immune-mediated inflammation after gelatin-impregnated graft, Gelweave Valsalva™ [Vascutek, Terumo, Renfrewshire, United Kingdom (UK)] and the newer generation of uncoated graft, Triplex™, aortic replacement.
Case Report
A 49-year-old man with hypertension who was otherwise healthy was brought by ambulance to our emergency room with sudden-onset left hemiplegia and dysarthria. Computed tomography (CT) revealed thoracic aortic dissection complicated by ischemic stroke. The patient underwent a modified Bentall procedure and total arch replacement. The modified Bentall procedure for aortic root replacement used a composite graft of a bioprosthetic aortic valve with a gelatin-coated woven Dacron vascular prosthesis (Gelweave Valsalva™). The ascending aorta and aortic arch were replaced with a three-layered uncoated knitted vascular prosthesis (Triplex™). Three days after surgery, he developed fever (38.5°C), malaise, nausea, and appetite loss. Three weeks later, these symptoms still continued. His vital signs were as follows: body temperature, 38.7°C; blood pressure, 151/79 mmHg; pulse, 102 beats/min; and respiratory rate, 16 breaths/min. The heart and lung sounds were normal. Tenderness or pus discharge was not observed at the surgical incision site. Other physical examination results were non-contributory. The serum CRP level was elevated to 6.1 mg/dL (normal range: <0.5 mg/dL). WBC count was 7400/mm3. Liver enzyme levels and kidney function were normal. Notably, pathological examination of the resected aorta revealed no evidence of aortitis. Repeated blood cultures revealed no bacterial growth. The patient’s condition remained stable without antibiotics during the first postoperative month. CT revealed pericardial and left pleural effusion, as well as a small amount of fluid collection around the grafts (Figure 1A). We made a presumptive diagnosis of post-pericardiotomy syndrome and administered colchicine (0.5 mg daily) and prednisolone (40 mg daily) 6 weeks postoperatively. The fever, malaise, and loss of appetite resolved, and CRP levels normalized. Pericardial and pleural effusions, as well as fluid collection around the aortic grafts, improved (Figure 1B). Colchicine was discontinued, and the prednisolone dose was tapered off 7 weeks after initiation. The overall disease course is shown in Figure 2.
Three weeks after discontinuation of prednisolone, his serum CRP level was again elevated at 8.0 mg/dL, although he remained afebrile and asymptomatic. One month later, malaise, hoarseness, and loss of appetite without fever developed. One month thereafter (6 months after admission), low-grade fever (37.6°C) and nausea developed. The CRP level continued to be elevated. WBC count was 5700/mm3. CT revealed recurrence of fluid collection around the aortic grafts (Figure 1C). An 18F fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed high uptake in the ascending aorta and arch, which was the location of the grafts (Figure 3), that is, both grafts of Gelweave Valsalva™ and Triplex™. The standardized uptake value (SUV) was 11.2 (Normal range: 2.1±0.5 [8]), suggesting inflammation around the implanted grafts. The repeated blood culture results were negative. He was presumptively diagnosed with immune-mediated periaortitis around the grafts and started on prednisolone 50 mg daily. All the above symptoms, including hoarseness, were resolved and the CRP level normalized (Figure 2).
Three weeks after starting prednisolone, he developed left-sided weakness and difficulty speaking and was brought to our emergency room by ambulance. He was diagnosed with acute ischemic stroke using brain magnetic resonance imaging. Contrast-enhanced CT scan revealed fluid collection with contrast leak into the periaortic space around the grafts (pseudo-aneurysm) and thrombus formation in the space (Figure 4). No new dissection was found in the carotid arteries. No pericardial or pleural effusions were observed. Transthoracic echocardiography showed a back-and-forth flow into the pseudoaneurysmal space at the anastomotic site of the left ventricular outflow and Gelweave Valsalva™ graft, demonstrating an anastomotic leak. A thrombus was also identified in the pseudoaneurysmal space on echocardiography, which was considered to be the etiology of the ischemic stroke. He underwent emergency aortic repair to prevent rupture. No pus was found during the surgery. Macroscopic findings showed that the peel did not adhere to the replaced grafts (both Gelweave Valsalva™ and Triplex™) (Figure 5), and cardioplegia solution was leaked into the pseudoaneurysmal space at the anastomotic site of the left ventricle outflow and Gelweave Valsalva™ graft (Figure 5), as well as the anastomotic site of the Gelweave Valsalva™ graft and Triplex™ graft. Of note, pathological analysis of the peel obtained in the second surgery showed only fibrotic change without inflammatory change, which was 3 weeks after initiation of prednisolone.
Methotrexate (8 mg) was added once weekly when the prednisolone dose was tapered to 40 mg. After the dose of prednisolone was decreased to 10 mg daily, it was tapered by 1 mg monthly (see Table 1 for detail dose of the therapy). Three months after starting prednisolone, a CT scan showed improvement of peri-graft fluid collection (Figure 1D). Prednisolone was discontinued 19 months after its initiation. Since then, the patient has been well on slow tapering of methotrexate once weekly without any evidence of recurrence of periaortitis for 1 year. We finally diagnosed him with non-infectious periaortitis induced by immune response to grafts based on the findings of elevated CRP levels, effusion around the grafts, high uptake around the aortic grafts on 18F FDG-PET scan, surgical findings of the dehiscence and anastomotic leak, nd excellent clinical response to glucocorticoid therapy, including resolution of fever, malaise, hoarseness, elevated CRP, and peri-graft fluid collection.
Discussion
We experienced a case of constitutional symptoms with elevated and CRP, peri-graft fluid collection persisting 6 months after aortic graft replacement, which was resolved by prednisolone. We initially considered 4 possible etiologies for the fever: aortic dissection, preceding Takayasu or other autoimmune aortitis, infection of the implanted grafts, and a non-infectious inflammatory reaction to the implanted grafts. The repeated blood culture results were negative. The patient’s condition showed marked improvement with glucocorticoid treatment without antibiotics. Therefore, an infection is unlikely.
Acute aortic dissection often precipitates fever during the acute phase [9]. Its mean duration is approximately 2 weeks, and fever usually improves spontaneously [9]. In our patient, the fever persisted for 6 months after aortic dissection and required immunosuppressive therapy for resolution. This protracted clinical course renders this an improbable culprit for his prolonged fever.
Non-infectious aortitis can develop fever and elevated CRP levels [10] and can cause aortic dissection [11]. This category encompasses Takayasu arteritis, giant cell arteritis, rheumatoid arthritis, systemic lupus erythematosus, Behçet’s disease, and immunoglobulin G4 (IgG4)-related disease, which usually cause manifestations beyond the aorta [11]. Isolated aortitis usually lacks constitutional symptoms or involvement of other organs [12] and requires pathological findings of surgical specimens for diagnosis [12]. Our patient was well until admission and had no signs or symptoms suggestive of these entities, such as fever, weight loss, joint pain, or skin or oral ulcers on admission. The surgical specimen showed no microscopic evidence of aortitis. Therefore, he was unlikely to have had non-infectious aortitis preceding aortic dissection.
Finally, after aortic graft replacement surgery, 74% of cases develop non-infectious inflammatory symptoms, specifically, fever, fatigue, elevated CRP, and peri-graft effusion [13]. One possible mechanism is an inflammatory reaction against bovine collagen or gelatin, which are impregnated in the inner layer of the grafts to prevent blood permeability [2–4]. They are usually degraded and absorbed within 1 month and then the inflammatory reaction resolves [1,5]. Another conceivable source of inflammation is endotoxin, which can be contaminated in bovine collagen-(Hemashield™ [5]) and gelatin-coated graft (Gelseal™ [14]), and can be pyrogenic and antigenic [5], although its detection in the Gelweave™ graft, coated with bovine gelatin, has not been reported yet. A newer generation graft, Triplex™, is a three-layered uncoated device. The inner and outer later layers are uncoated knitted polyester. The middle layer is self-sealing elastomeric membrane [15]. This design includes no animal-derived material, theoretically reducing the risk of early-phase inflammatory reactions [6]. However, a study showed that Triplex™ can develop an inflammatory response of elevated body temperature, elevated WBC count, and high CRP, which did not significantly differ from those associated with use of collagen-coated grafts [15]. In contrast, other studies showed significantly lower inflammatory responses compared to gelatin- and collagen-coated grafts [6,16], although the response was reportedly resolved at postoperative day 5 in case of Triplex™ [15], a shorter duration than observed with collagen- or gelatin-coated grafts.
The inflammation around grafts can persist for longer than 1 month, as in our case [17–20], although it is rare. The precise mechanism of prolonged inflammation is unclear. Given that prednisolone effectively suppressed fever, elevated CRP, and peri-graft fluid collection in our case, the inflammatory presentation was likely immune-mediated. A possible mechanism underlying the immune reaction is antibody production against the graft’s coating material [21]. A study showed that antibody against bovine collagen type I was detected in 5 out of 68 patients after aortic graft replacement [21]. Although collagen is usually absorbed soon after implantation, a small amount may remain at 10 months after replacement [22]. No studies have investigated antibody against gelatin impregnated in Gelweave™ and antibody against the material included in Triplex™. Further research is needed to determine the precise mechanism of the prolonged immune reaction, as in our patient, such as antibody production against gelatin or other material included in Gelweave™ and Triplex™, as well as cell-mediated immune reaction like lymphocyte proliferation induced by material incorporated into these grafts.
On FDG-PET scans, FDG accumulation is observed in both infectious and non-infectious arteritis. Diffuse uptake was identified in 92% of non-infected vascular prosthetic grafts and its intensity did not change over several years without any associated symptoms [23]. SUV ranged between 0.4 and 6.3 (average 1.9 for all prosthetic grafts and 2.35 for Dacron grafts) [23]. Our patient had high uptake around the ascending aorta and arch, which was the location of the grafts (Gelweave Valsalva™ and Triplex™) with SUV 11.2, much higher than the above reference level, suggesting inflammation around both implanted grafts.
Our patient had fluid accumulation around both the Gelweave Valsalva™ and Triplex™ grafts, which resolved after the initiation of prednisolone. This clinical course suggested inflammatory reaction against both grafts. Notably, he developed hoarseness 5 months after the first surgery, making the usual post-surgical inflammation unlikely. His hoarseness resolved and did not recur with treatment with prednisolone and methotrexate, suggesting that the cause of hoarseness was recurrent nerve palsy due to periaortitis at the aortic arch, probably through an immune-mediated inflammatory reaction against the Triplex™ graft.
Non-infectious inflammation around grafts generally resolves within 1–2 weeks and within 1 month at the longest [2–4,7]. To the best of our knowledge, there have been 5 reported cases of aortic graft replacement, including ours, who developed non-infectious inflammatory persisting for more than 30 days (Table 2) [17–20]. Of those, 3 required glucocorticoid treatment, including our case [18,20]. To the best of our knowledge, there has been no such case caused by a Triplex™ graft, and our case appears to be the first reported.
After aortic graft replacement surgery, anastomotic pseudoaneurysm and graft detachment can occur, albeit rarely. Its mechanism other than infection is still unknown [24]. Takayasu arteritis can be complicated with aortic valve regurgitation and aortic aneurysm, requiring aortic valve replacement and aortic graft replacement. After surgery, detachment of prosthetic valve and anastomosis of the prosthetic graft occurred in 11.1% and 3.7% of cases, respectively [25]. Active inflammation was found to be a risk factor [25], although the detachment also could occur as a result of fragility of the aortic wall or annular tissue without tissue inflammation caused by Takayasu arteritis [25]. Immunosuppressive treatment with glucocorticoid is suggested for the prevention of these complications [25]. There is also a report of successful resolution of anastomotic pseudoaneurysm developed after aortic graft replacement for aortic aneurysm due to Behçet’s aortitis with glucocorticoid treatment [26]. This observation suggested that immune-mediated inflammation can cause detachment of the implanted prosthetic valve or graft, or anastomotic aneurysm formation.
The management of persistent non-infectious periaortitis after aortic replacement surgery is not yet established. Therefore, to manage our patient we referred to that of aortitis. A meta-analysis of giant cell arteritis (GCA) demonstrated that methotrexate in conjunction with glucocorticoid reduced the risk of relapse, increased the probability of glucocorticoid-free remission for over 24 weeks, and lowered the cumulative glucocorticoid dose at week 48 in patients treated with methotrexate versus placebo [27]. Consequently, methotrexate is recommended as an adjunctive therapy for patients with GCA, especially those with refractory or relapsing disease and concerns about glucocorticoid-related adverse effects [28]. Accordingly, we considered methotrexate as a potential glucocorticoid-sparing agent for our patient.
The treatment of anastomotic pseudoaneurysm after thoracic aortic graft replacement surgery is usually surgical repair. However, redo thoracic aortic surgery has a high risk of complication and death. To avoid these, endovascular stent-grafting, which is a less invasive procedure, has been introduced for the descending thoracic aorta or aortic arch [29]. In our case, because anastomotic pseudoaneurysm developed close to the prosthetic aortic valve and we could not place a stent graft, we chose surgical repair.
This report has certain limitations. First, our diagnosis of immune-mediated periaortitis was solely based on the patient’s good response to glucocorticoid treatment. To substantiate this, future research should investigate immunological testing, such as evaluation of antibody against bovine gelatin and other material in the grafts, checking endotoxin level in the peri-graft fluid and antibody against endotoxin, and lymphocyte proliferation testing by the material included in the graft. In making the diagnosis, we did not measure the IgG4 level. Although IgG4 syndrome-related aortitis may be a possible diagnosis, it usually does not cause fever or elevated CRP [30], and pathology of the resected aorta did suggest aortitis; therefore, it is unlikely.
Conclusions
We report a case of inflammatory reaction to thoracic aortic grafts using Gelweave™ and Triplex™, probably through an immune-mediated mechanism, persisting for 6 months, leading to serious complications, requiring long-term predniso-lone and methotrexate. When fever persists for longer than 1 month after prosthetic graft replacement surgery for aortic dissection, the clinician should consider an immune response against the grafts, which requires close monitoring for dehiscence of grafts and anastomotic leak by imaging studies and timely initiation of immunosuppressive treatment to prevent serious complications.
Figures
References:
1.. Hirt SW, Aoki M, Demertzis S, Comparative in vivo study on the healing qualities of four different presealed vascular prostheses: J Vasc Surg, 1993; 17(3); 538-45
2.. Lacroix H, Boel K, Nevelsteen A, Suy R, Early inflammatory response to gelatin- and collagen-sealed Dacron prostheses: Ann Vasc Surg, 1995; 9(2); 152-54
3.. Kawashima T, Kamisawa O, Fuse K, [Postoperative inflammatory responses to gelatin- and collagen-impregnated Dacron grafts and changes of endotoxin]: Nihon Kyobu Geka Gakkai Zasshi, 1997; 45(4); 531-35 [in Japanese]
4.. Utoh J, Miyauchi Y, Goto H, Inflammatory reactions after vascular pros-thesis implantation: A comparison of gelatin-sealed and unsealed Dacron prostheses: Surg Today, 1996; 26(4); 258-61
5.. Yamamoto K, Noishiki Y, Mo M, Unusual inflammatory responses around a collagen-impregnated vascular prosthesis: Artif Organs, 1993; 17(12); 1010-16
6.. Ohata T, Ueda H, Kobayashi K, Terumo-Triplex grafts for total arch replacement: analysis of postoperative graft performance: J Artif Organs, 2012; 15(3); 240-43
7.. Shindo S, Motohashi S, Katsu M, Coated prostheses are associated with prolonged inflammation in aortic surgery: A cost analysis: Artif Organs, 2008; 32(3); 183-87
8.. Stellingwerff MD, Brouwer E, Lensen KDF, Different scoring methods of FDG PET/CT in giant cell arteritis: Need for standardization: Medicine (Baltimore), 2015; 94(37); e1542
9.. Shimada S, Nakamura H, Kurooka A, Fever associated with acute aortic dissection: Circ J, 2007; 71(5); 766-71
10.. Cozijnsen L, Ter Borg EJ, Braam RL, Ascending aortic aneurysm secondary to isolated noninfectious ascending aortitis: J Clin Rheumatol, 2019; 25(4); 186-94
11.. Gornik HL, Creager MA, Aortitis: Circulation, 2008; 117(23); 3039-51
12.. Bossone E, Pluchinotta FR, Andreas M, Aortitis: Vascul Pharmacol, 2016; 80; 1-10
13.. Yao YT, Li LH, Lei Q, Noninfectious fever following aortic surgery: Incidence, risk factors, and outcomes: Chin Med Sci J, 2009; 24(4); 213-19
14.. Ohshima N, Yamada T, Nakahara H, Sealed versus non-sealed Dacron knitted prosthesis: Comparison of intraoperative blood loss and inflammatory response: Jpn J Artif Organs, 1994; 23(3); 825-28
15.. Fukunaga N, Matsuo T, Koyama T, Does triplex vascular prosthesis contribute to reducing the inflammatory reaction after surgical repair of abdominal aortic aneurysms?: Ann Vasc Dis, 2016; 9(2); 91-94
16.. Tamura A, Yamaguchi A, Yuri K, Clinical experience with a new vascular graft free from biodegradable material: Interact Cardiovasc Thorac Surg, 2011; 12(5); 758-61
17.. Nantsios A, Rubens FD, Unexplained fever following DeBakey type I aortic dissection repair with woven Dacron grafts: J Card Surg, 2021; 36; 2175-78
18.. Chiba Y, Muraoka R, Ihaya A, Postoperative inflammatory reactions of impregnated Dacron grafts: Surg Today, 1999; 29(11); 1225-28
19.. Ishikawa S, Ohtaki A, Takahashi T, Non-infective high fever after replacement of thoracic aorta using collagen-impregnated Dacron prosthesis: J Cardiovasc Surg (Torino), 1995; 36(2); 143-45
20.. Uchida N, Yamazaki M, A case of inflammatory abdminal aortic aneurysm whose persistent postoperative high fever was succesfullty treated by steroid: Jpn J Cardiovasc Surg, 2003; 32; 132-36
21.. , Immunologic response to collagen-impregnated vascular grafts: A randomized prospective study: J Vasc Surg, 1990; 12(6); 741-46
22.. Helfer E, Kuntz S, Dion D, Vascular grafts collagen coating resorption and healing process in humans: JVS Vasc Sci, 2022; 3; 193-204
23.. Keidar Z, Pirmisashvili N, Leiderman M: J Nucl Med, 2014; 55(3); 392-95
24.. Kalapatapu VR, Shelton KR, Ali AT, Pseudoaneurysm: A review: Curr Treat Options Cardiovasc Med, 2008; 10(2); 173-83
25.. Matsuura K, Ogino H, Kobayashi J, Surgical treatment of aortic regurgitation due to Takayasu arteritis: Long-term morbidity and mortality: Circulation, 2005; 112(24); 3707-12
26.. Naraoka S, Uchiyama H, Yano T, Pseudorepair of pseudoaneurysm?: Front Cardiovasc Med, 2021; 8; 683216
27.. Mahr AD, Jover JA, Spiera RF, Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis: Arthritis Rheum, 2007; 56(8); 2789-97
28.. Hellmich B, Agueda A, Monti S, 2018 Update of the EULAR recommendations for the management of large vessel vasculitis: Ann Rheum Dis, 2020; 79(1); 19-30
29.. Ito T, Kurimoto Y, Kawaharada N, Endovascular stent-grafting of anastomotic pseudoaneurysms following thoracic aortic surgery: Gen Thorac Cardiovasc Surg, 2009; 57(10); 528-33
30.. Stone JH, Zen Y, Deshpande V, IgG4-related disease: N Engl J Med, 2012; 366(6); 539-51
Figures
Tables
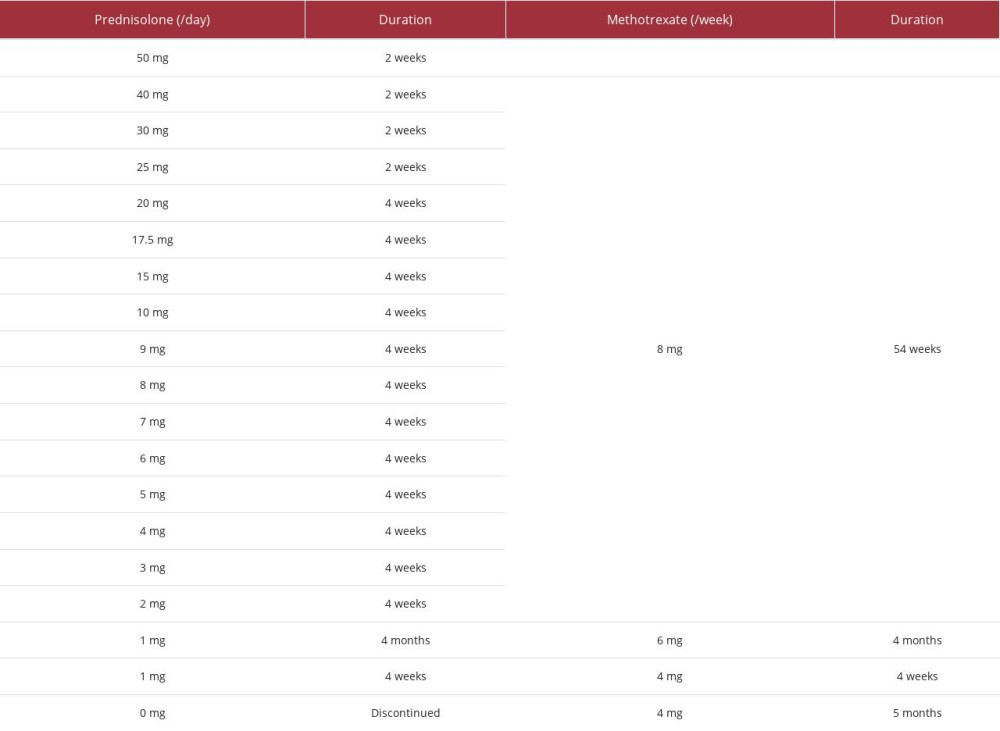 Table 1.. Detail treatment course of prednisolone and methotrexate.
Table 1.. Detail treatment course of prednisolone and methotrexate.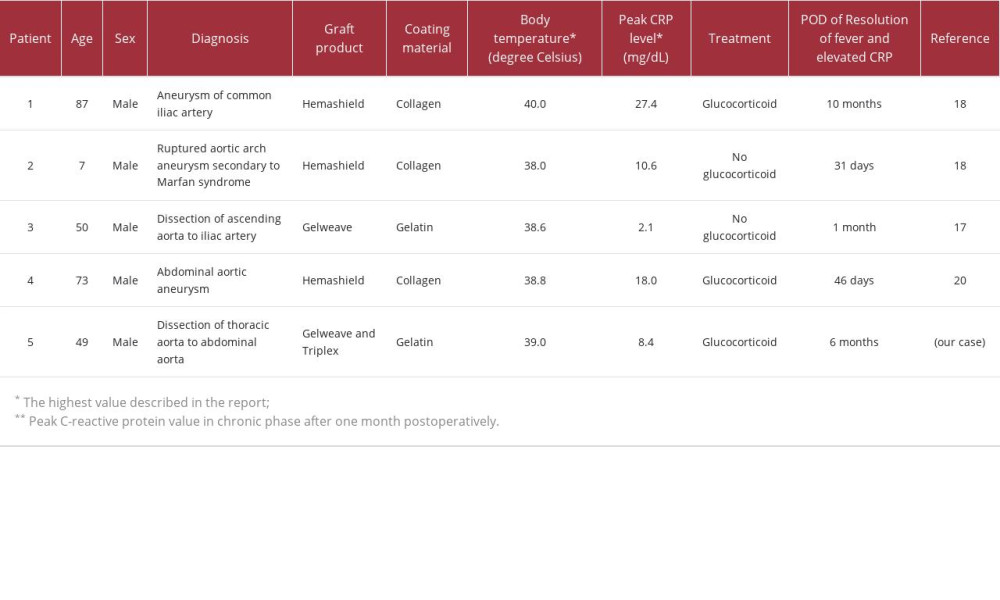 Table 2.. Cases of non-infectious inflammatory reaction (fever, elevated C-reactive protein, and peri-graft effusion) persisting for longer than 1 month.
Table 2.. Cases of non-infectious inflammatory reaction (fever, elevated C-reactive protein, and peri-graft effusion) persisting for longer than 1 month. Table 1.. Detail treatment course of prednisolone and methotrexate.
Table 1.. Detail treatment course of prednisolone and methotrexate. Table 2.. Cases of non-infectious inflammatory reaction (fever, elevated C-reactive protein, and peri-graft effusion) persisting for longer than 1 month.
Table 2.. Cases of non-infectious inflammatory reaction (fever, elevated C-reactive protein, and peri-graft effusion) persisting for longer than 1 month. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








