11 January 2024: Articles 
DRESS Syndrome: Renal Involvement in Two Cases – A Comprehensive Analysis and Literature Review of Improved Diagnosis and Treatment
Rare disease, Adverse events of drug therapy
Magdalena Natalia Mąsior1EF, Olga Maria RostkowskaDOI: 10.12659/AJCR.942315
Am J Case Rep 2024; 25:e942315
Abstract
BACKGROUND: Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare hypersensitivity reaction involving the skin and various visceral organs; the kidneys are the second most affected organ. Many drugs are reported to be associated with DRESS, particularly antiepileptic agents and allopurinol. Certain human leukocyte antigen (HLA) haplotypes, in combination with a particular drug, can further contribute to an increased risk of DRESS. Symptoms often develop 2 to 8 weeks after drug initiation. If diagnosis is delayed, DRESS can be a life-threatening condition.
CASE REPORT: We present cases of 2 patients. The first patient was an 86-year-old Polish woman who developed acute kidney injury and skin lesions with accompanying leucocytosis and eosinophilia during long-term antibiotic therapy with piperacillin/tazobactam and ciprofloxacin. The second patient was a 37-year-old Asian woman with predialysis chronic renal disease stage V in the course of IgA nephropathy. Two weeks after starting allopurinol in a standard dose, she presented with maculopapular rash, facial edema, fever, liver injury, and eosinophilia. Renal function started to deteriorate, but she did not require dialysis. In both cases, the discontinuation of the above-mentioned drugs and the introduction of steroid therapy and intravenous immunoglobulins allowed for clinical improvement and recovery. In the second case, the extended 4-locus HLA typing was performed retrospectively, and allele HLA-B*5801 was found.
CONCLUSIONS: Due to the rare occurrence and heterogeneous manifestation of DRESS, its diagnosis can pose many difficulties. In-depth analysis of symptoms, medicines taken, and laboratory findings enable the implementation of appropriate treatment and recovery.
Keywords: Allopurinol, Drug Hypersensitivity Syndrome, Piperacillin, Tazobactam Drug Combination, Renal Insufficiency, Chronic
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, also referred to as drug-induced hypersensitivity syndrome, is a rare hypersensitivity reaction that involves the skin and various visceral organs, such as the liver, kidneys, lungs, and heart. DRESS prevalence is estimated at 2 per 100 000 patients per year [1]. Approximately 75% of DRESS cases are caused by a few high-risk drugs, such as allopurinol, aromatic anticonvulsants, and sulphonamides [2]. The occurrence of DRESS can also be connected with several human leukocyte antigen (HLA) haplotypes [3] and genetic variants [4]. Reactivation of infections caused by herpes viruses, especially HHV-6, is a phenomenon often associated with DRESS [5]. DRESS typically occurs 2 to 8 weeks after the initiation of the culprit drug. The symptoms include skin lesions (dermatitis) and non-specific prodromal symptoms, such as fever or lymphadenopathy, and more specific symptoms, such as generalized rash with facial edema, and involvement of one or more internal organs, with the liver being affected most frequently. The kidney is the second most affected visceral organ. This can be determined by elevated serum creatinine (SCr) levels, decreased glomerular filtration rate (GFR), or hematuria, proteinuria, and eosinophiluria. [6] The differential diagnosis of DRESS syndrome includes other cutaneous adverse drug reactions, such as Stevens-Johnson syndrome/toxic epidermal, necrolysis autoimmune diseases, such as acute cutaneous lupus erythematosus, and lymphomas, hypereosinophilic syndromes, and viral infections. In the case of renal involvement, it is important to differentiate DRESS from drug-induced acute interstitial nephritis, which often manifests with the triad of rash, fever, and eosinophilia. DRESS is usually accompanied by involvement of other internal organs and features, such as lymphadenopathy and facial swelling, that are absent in interstitial nephritis [6]. The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) scoring system is used to confirm or exclude the diagnosis of DRESS [2].
Below we present 2 case reports on DRESS. In the first case, DRESS was induced by the extremely rare culprit drugs piperacillin/tazobactam (Pip/Taz) and ciprofloxacin. In the second case, a patient with end-stage renal failure experienced DRESS and uneventfully underwent renal transplantation a year later.
Case Reports
CASE 1:
An 85-year-old Polish woman was admitted to the hospital because of a multi-level proximal femoral fracture. She was taking the following medications on a regular basis: valsartan, indapamide, bisoprolol, metformin, gliclazide, and potassium supplementation. She denied any allergies, including to drugs. No previous autoinflammatory diseases were noted in the medical records. The patient was treated surgically with open reduction internal fixation with a gamma nail. The perioperative period was uneventful, and she was discharged from the orthopedic ward. After 4 weeks, the patient was admitted to the hospital for surgical site infection, and empirical therapy was initiated with intravenous (i.v.) ciprofloxacin. A culture grown from the wound revealed
In the fourth week of hospitalization, the general condition of the patient began to deteriorate. She developed diarrhea and low-grade fever. In the following days, she developed a rash with edema, initially affecting her face only. Her laboratory tests were suggestive not only of inflammation, with CRP concentration of 198 mg/L, WBC count of 16 000/µL, and neutrophil count of 9350/µL (58% of all leucocytes), but also of progressive renal function deterioration, with the increase of SCr from 0.8 mg/dL to 3.7 mg/dL. The estimated glomerular filtration rate (eGFR) using CKD-EPI dropped from 67 mL/min/1.73 m2 to 11 mL/min/1.73 m2. The blood count was also significant for eosinophil count of 5430/µL (21% of all WBC). Liver enzymes remained stable and within the reference range. Urinalysis showed leukocyturia and significant proteinuria (greater than 1 g daily). The following day, the laboratory test results showed increasing inflammatory markers: CRP concentration of 200 mg/L and procalcitonin of 46 ng/mL (with reference of 0.5 ng/mL), eosinophil count of 7000/µL (35% of all WBC), leukocyte count of 20 000/µL, and neutrophil count of 11 000/µL (55% of all WBC). Diuresis began to decrease, while sCr increased up to 4.2 mg/dL. Because of diarrhea and suspected Clostridioides difficile infection, it was decided to discontinue the previously taken antibiotics. The next day, the edema spread to both upper limbs, the trunk, and the previously operated lower limb. Diffuse erythemathous skin lesions with discreet scaling covered most surface of the body, especially the face, neck, and chest (Figure 1). Oral or conjunctival mucosa were not involved. The patient was confused, and logical and verbal contact became limited. Serum creatinine reached 4.5 mg/dL (eGFR 8 mL/min/1.73 m2), diuresis was 1300 mL, with a fluid intake of 2300 mL. Eosinophils increased further to 9500/µL (42% of all leukocytes). Urine, blood, and stool microbiological cultures were negative. At this point, due to eosinophilia and skin lesions accompanied by renal function deterioration, DRESS syndrome was suspected, and steroid therapy was started: i.v. methylprednisolone in a dose of 1 mg/kg of total body weight, initially at 70 mg once daily. The patient was also treated with clobetasol propionate topically twice a day. Shortly after methylprednisolone therapy was initiated, the patient developed herpes simplex virus (HSV) infection on her lips (Figure 2), which was successfully treated with acyclovir administered orally (p.o.). In the following days, improvement of the patient’s condition and normalization of the inflammation markers were observed. Methylprednisolone was initially administered at a dose of 70 mg, then reduced to 60 mg. As the significant subside of skin lesions was observed during the course of the therapy, after 8 days, the intravenous steroid was switched to prednisone p.o. Applied glucocorticoids and fluid therapy with furosemide resulted in improvement in renal function in terms of diuresis and SCr concentration. Upon discharge from the hospital, the SCr level was 1.4 mg/dL (eGFR 34 mL/min/1.73 m2), with the dose of prednisone at 20 mg p.o. The patient was referred to her general practitioner. There was no relapse.
CASE 2:
A 37-year-old Vietnamese woman was admitted to our hospital because of an acute maculopapular rash involving more than 50% of her body surface, which had appeared 1 week earlier. Other symptoms included sore throat, headache, and fever. Two weeks before admission to the hospital, she had started allopurinol in a dose of 100 mg once daily due to asymptomatic hyperuricemia. Upon a dermatological consultation, allopurinol was discontinued, and the patient was prescribed prednisone at a dose of 15 mg p.o. once daily and a steroid ointment, but no improvement was observed. The medical history of the patient was significant for end-stage renal disease due to chronic glomerulonephritis/IgA nephropathy. She had been awaiting preemptive renal transplantation. The patient had no previous history of autoimmune diseases. Her home medications included sodium bicarbonate, bisoprolol, nitrendipine, and calcium bicarbonate. Upon admission, the patient presented with skin involvement, including the disseminated erythematous and erythematous-papular lesions located in particular on the trunk and limbs, as well as the presence of erosions on the lips and conjunctival redness (Figure 3). Other physical examination findings were insignificant. The laboratory test results revealed a neutrophil count of 8710/µL (82% of all WBC), without signs of leukocytosis or eosinophilia, and increased levels of SCr, at 6.4 mg/dL, and urea, at 161 mg/dL (1 year prior to the episode described, those parameters were 1.5 mg/dL and 49 mg/dL, respectively). There were also laboratory signs of liver damage, with alanine transaminase activity (ALT) level of 396 IU/L, aspartate transaminase level (AST) of 216 IU/L, and gamma-glutamyltransferase level of 76 IU/L. Among inflammation markers, procalcitonin was elevated, at 2.71 ng/mL, and CRP was 8.1 mg/L. Ultrasound of the abdomen and radiography of the chest were insignificant.
Following dermatological consultation, prednisone therapy was discontinued, and dexamethasone was started intramuscularly at a dose of 8 mg once daily. Skin and mucosal lesions were treated topically. Ceftriaxone was started on the second day of the hospitalization. The delay was caused by the suspected allergic reaction.
On the second day of treatment, edema of the lips, tongue, eyelids, hands, and genitals appeared, accompanied by erosions of the lips and oral mucosa. The patient developed leucocytosis with a leucocyte count of 13 530/µL, and neutrophilia with neutrophil count of 10 600/µL (78% of all leucocytes). The eosinophil count remained normal (300/µL). Fluid intake and urination became difficult due to swollen lips and tongue as well as the genital area, so a Foley catheter was inserted, and the patient was given the following i.v. medications: hydrocortisone (200 mg), clemastine (2 mg) and calcium. However, cardiovascular parameters, including blood pressure and heart rate, were stable.
The next day, the response to treatment was poor: the skin lesions with edema persisted, and the values of procalcitonin and ALT were increasing, up to 4.1 ng/mL and 653 IU/L, respectively. Eosinophils of 1330/µL was also observed. DRESS was suspected at this point. Glucocorticosteroid therapy was modified, and dexamethasone was replaced with methylprednisolone at a dose of 1 mg/kg of body weight (maximal applied dose: 60 mg daily), with gradual dose reduction based on the patient’s condition. Additionally, a decision was made to start simultaneous intravenous immunoglobulins (IVIG) in a dose of 2 to 3 g/kg of body weight (doses of 40 g, 35 g, and 30 g on 3 consecutive days), but the treatment was uneventful. The patient was also prescribed acyclovir in a dose of 200 mg 3 times a day to prevent the reactivation of viruses from the Herpesviridae family. The dose was adjusted for renal insufficiency.
Additionally, a skin biopsy was performed, revealing interface dermatitis with unclear skin infiltration. Further diagnostic tests were performed, including autoimmune and infectious causes as potential differential diagnoses. Antinuclear antibodies, antibodies to neutrophil cytoplasm, and antibodies to double-stranded DNA were all negative. Microbiological samples of blood, urine, and stool cultures did not present any growth. Cytomegalovirus (CMV), Epstein-Barr virus, severe acute respiratory syndrome coronavirus 2, and hepatitis C virus PCR tests were negative. Hepatitis B tests indicated past infection.
During the next 2 weeks of hospitalization, a gradual reduction in the severity of skin lesions with accompanying scaling was observed. The facial edema subsided, and the erosions in the oral mucosa began to heal. Clinical improvement was accompanied by a decrease in liver parameters (ALT and AST levels at 119 U/L and 39 U/L, respectively), procalcitonin (from 4.1 ng/mL to 0.4 ng/mL), CRP (from 15 mg/L to 0.7 mg/L), eosinophils (from 2200/µL to 480/µL), and creatinine (from 6.4 to 3.6 mg/dl). Methylprednisolone was given at a dose of 60 mg for 6 days, reduced to 40 mg for further 4 days, followed by the dose of 20 mg for the final 6 days of i.v. treatment. The steroid was then changed to prednisolone at 20 mg p.o. daily with a gradual reduction of the dose. The patient was regularly consulted by an ophthalmologist due to pseudomembranous conjunctivitis. Following the introduction of ofloxacin-dexamethasone combination eye drops and regular sterile mechanical removal of pseudomembranes, improvement was achieved within 18 days. The patient was discharged from the hospital after 22 days. Four days after the discharge, the patient presented with a minor skin flare-up, which was successfully treated in a dermatological outpatient clinic. She was given strict recommendations not to use allopurinol lifelong. Within a year, the patient underwent uneventful renal transplantation. The post-transplant period was uncomplicated, and the IgA nephropathy did not relapse. For the purpose of this article, an extended 4-locus HLA typing was performed using a DNA-based method, where allele HLA-B*5801 was found.
Discussion
DRESS belongs to the group of delayed hypersensitivity reactions to drugs. The Gell and Coombs classification assigns DRESS to group IVb, in which T-cell mediated eosinophilic inflammation dominates the pathogenesis and the symptoms appear after 2 to 6 weeks. The latency periods in our patients agreed with this typical chronology of the drug reaction [7]. It should be noted that there is another mechanism causing drug-induced eosinophilia: type I hypersensitivity reaction, in which immunoglobulin E-dependent degranulation of mast cells and/or basophils is observed. Characteristic symptoms, such as anaphylaxis or bronchospasms, appear within 1 to 6 h after drug initiation [8].
The literature is mostly based on case series or reports on individual patients in whom DRESS developed as an adverse reaction to a specific medicine. In case of some culprit drugs, such as antitubercular medications or vancomycin [9], certain characteristic features could be observed, for example, the latency period, most common organ involvement, or prognosis.
There are only a few case reports, including case series by Cabañas et al [10], about DRESS caused by Pip/Taz [11–13]. Pip/Taz was also one of the most common culprit drugs in a single-center retrospective study by Del Pozzo-Magaña et al [14]. Ciprofloxacin was described as a culprit drug only 4 times in the literature [15–18]. Some authors observed a shorter than usual symptom onset (2 days), which is typical for ciprofloxacin-induced DRESS syndrome [17,18]. In terms of Pip/Taz-induced DRESS, a mean latency period of 18 days and 28 days was reported by Cabañas et al and Del Pozzo-Magana et al, respectively. In our case, the latency period lasted 28 days and 39 days for Pip/Taz and ciprofloxacin, respectively.
Allopurinol belongs to a group of medicines that causes DRESS relatively often [19], especially in women, patients with cardiovascular diseases, asymptomatic uricemia, and renal insufficiency [20,21], which was also observed in our patient with chronic kidney disease and other above-mentioned risk factors. Chung et al reported that renal failure significantly reduces the clearance of oxypurinol, the allopurinol metabolite. Consequently, metabolite accumulation induces a cytotoxic T-cell response, the main cause of eosinophilic inflammation in DRESS syndrome. Increased oxypurinol plasma levels are also linked to prolonged cutaneous reactions in allopurinol-induced DRESS [22].
The strong association of the allele HLA-B*5801 with the risk for allopurinol-induced DRESS, Stevens-Johnson syndrome, and toxic epidermal necrolysis was first reported by Shuen-Iu et al [23]. Their trial was performed on a population of Han Chinese, but the authors pointed out that the HLA-B*5801 extended haplotype can also be present in other Asian populations. The strong association between the presence of the HLA-B*5801 allele and the potential risk of DRESS was reiterated by Zhihao et al [24]. An explanation for this association may be the increased affinity of allopurinol or its metabolite oxypurinol for the HLA-B*58: 01 peptide binding groove, which was performed in silico [25]. As described in our case 2, the patient of Vietnamese origin presented with the HLA-B*5801 allele.
The Adverse Drug Reaction probability scale, often referred to as the Naranjo scale, is a widely used method for assessing the probability of a causal sequence between a clinical event and a drug [26]. According to this questionnaire, the score in the first event was 3 points (for Pip/Taz), meaning that the causal sequence was “possible”. In case 2, it was 7 points, meaning that the reaction was “probably” caused by the drug.
Liver involvement is the most frequent internal-organ manifestation of DRESS [2]. Both of our patients developed acute kidney injury (AKI). This stands in opposition with the observation from case 1 that in Pip-Taz-induced DRESS the liver is the most frequently affected organ [11]. DRESS with renal involvement is more often induced by allopurinol, as was also observed in case 2 [27]. The incidence of renal involvement during DRESS varies depending on the source, from 5% to 65%, but in most retrospective studies it ranges from 15% to 35%. Dagnon da Silva et al [6] in their systemic review report AKI as the most frequent renal manifestation (96%). The isolated SCr elevation was the most common, while oliguria, anuria, proteinuria, or hematuria accompanied AKI less frequently. Isolated proteinuria and/or hematuria, without AKI, were observed in less than 5% of cases. About 30% of patients required temporary renal replacement therapy and were usually fully recovered; there was only 1 described case of ethambutol-induced progression to chronic kidney disease requiring long-term renal replacement therapy [28] and 1 case of quetiapine-induced permanent renal function deterioration [29]. The mortality rate in DRESS with renal involvement was 13%, which was higher than in DRESS overall (10%) [6]. In case 1, the patient developed AKI and significant proteinuria (>1 g) with leukocyturia. The applied treatment resulted in a gradual improvement in kidney function, with normalization of SCr and GFR to the baseline level. In the case with allopurinol-induced DRESS, the patient developed exacerbation of chronic kidney disease, with SCr at 6.4 mg/dL, which gradually decreased during proper treatment to 3.6 mg/dL.
Both patients developed skin rash involving over 50% of the body surface area and facial edema. In the case of allopurinol-induced DRESS, the patient also had pseudomembranous conjunctivitis, which has been reported in other cases as a rare form of mucosal involvement [30]. The diagnosis of DRESS in both presented cases was definite based on the RegiSCAR scoring system. In case 2 (allopurinol), the score was 8, and in case 1 (Pip/Taz and ciprofloxacin), it was 6 points. The diagnosis of DRESS is made with at least 6 points (Table 1).
One of our patients developed herpes simplex labialis about 10 days after the onset of skin eruptions. There are reports in the literature that viruses from the Herpesviridae family can reactivate in association with DRESS. HHV-6 reactivation, as the most common one, is included in the diagnostic criteria for drug-induced hypersensitivity syndrome established by a Japanese consensus group but not in the RegiSCAR criteria. It remains unclear how this phenomenon contributes to DRESS pathogenesis, but HHV-6 reactivation is considered a risk factor for the severe course of the disease [5,31]. CMV, HHV-7, or Epstein-Barr virus reactivation can also occur [32]; however, we found only 1 case describing DRESS coinciding with genital ulcers caused by HSV, accompanied by the reactivation of HHV-6 and CMV [33]. In our case, diagnostics for herpes viruses other than HSV were not performed. In our case 2, of allopurinol-induced DRESS, the infectious diagnostics included CMV and EBV but not HHV-6. Both of our patients would have potentially benefitted from the extension of diagnostics.
The mortality rate due to DRESS is estimated to be up to 10%, with severe organ involvement and multiorgan failure considered as the leading causes of death according to the literature [2,34]. Patients in both of our cases survived, but the course of the syndrome was severe and required aggressive therapy. Knowledge about the treatment of DRESS is mostly case-based, and the clinical trials, as well as evidence-based guidelines, are lacking. Prompt withdrawal of the culprit drug definitely plays the most important role. Glucocorticosteroids are the first-line therapy, depending on the severity of symptoms, and either a topical or systemic form can be used [35]. Due to a satisfying response to therapy, the first patient was treated only with methylprednisolone. Combined treatment of systemic steroids and IVIG, as in our allopurinol-induced DRESS, remains second-line therapy. The exact mechanism of action of IVIG remains unclear but may be related to their multidirectional immunologic effects and the presence of antiviral IgG [36]. The remaining therapeutic options include cyclosporine A and other immunosuppressive agents, such as IL-5 antagonist mepolizumab [16] and IL-5 receptor blocker benralizumab [37]. At the point of submitting the article, we found no other therapies in line.
In case 1, the administration of Pip/Taz and ciprofloxacin was absolutely contraindicated for life. In case 2, there was a lifetime contraindication to use allopurinol. Other pharmacological options to treat asymptomatic hyperuricemia are not available due to the patient’s renal dysfunction. Therefore, appropriate lifestyle intervention plays a key role in the patient from case 2.
The limitations of our study include the lack of skin biopsy in case 1. Any tests to confirm the drug causality were not available at our center at the time of hospitalization of both patients.
Conclusions
Due to its rare occurrence and heterogeneous manifestation, diagnosis of DRESS can pose many difficulties. In-depth analysis of symptoms, medicines used, and laboratory test results enable the implementation of appropriate treatment facilitating recovery. The identification and discontinuation of the culprit drug plays a key role. In both of our cases, the kidneys were affected in the course of DRESS, which is a common occurrence in this condition.
Figures
References:
1.. Wolfson AR, Zhou L, Li Y, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome identified in the electronic health record allergy module: J Allergy Clin Immunol Pract, 2019; 7(2); 633-40
2.. Kardaun SH, Sekula P, Valeyrie-Allanore L, Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study.: Br J Dermatol, 2013; 169(5); 1071-80
3.. Konvinse KC, Trubiano JA, Pavlos R, HLA-A*32: 01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms: J Allergy Clin Immunol, 2019; 144(1); 183-92
4.. Chung WH, Chang WC, Lee YS, Genetic variants associated with phenytoin-related severe cutaneous adverse reactions: JAMA, 2014; 312(5); 525
5.. Tohyama M, Hashimoto K, Yasukawa M, Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome: Br J Dermatol, 2007; 157(5); 934-40
6.. Dagnon da Silva M, Domingues SM, Oluic S, Radovanovic M, Renal manifestations of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome: A systematic review of 71 cases: J Clin Med, 2023; 12(14); 4576
7.. Schrijvers R, Gilissen L, Chiriac AM, Demoly P, Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back: Clin Transl Allergy, 2015; 5; 31
8.. Roberts CE, Mortenson LY, Merrill DB, Successful rechallenge with clozapine after eosinophilia: Am J Psychiatry, 2011; 168(11); 1147-51
9.. Sharifzadeh S, Mohammadpour AH, Tavanaee A, Elyasi S, Antibacterial antibiotic-induced drug reaction with eosinophilia and systemic symptoms (dress) syndrome: A literature review: Eur J Clin Pharmacol, 2020; 77(3); 275-89
10.. Cabañas R, Calderon O, Ramirez E, T. Piperacillin-induced DRESS: Distinguishing features observed in a clinical and allergy study of 8 patients.: J Investig Allergol Clin Immunol, 2014; 24(6); 425-30
11.. Bai M, Govindaraj V, Kottaisamy R, Vijayarangam N, Drug reaction with eosinophilia and systemic symptoms syndrome related to piperacillin-tazobactam use: J Postgrad Med, 2022; 68(2); 102-5
12.. Song G, Cheng MQ, Li R, Drug-induced hypersensitivity syndrome with high procalcitonin levels due to piperacillin/tazobactam and meropenem: A case report: Front Med (Lausanne), 2022; 9; 951714
13.. Sanchez-Gonzalez MJ, Barbarroja-Escudero J, Antolin-Amerigo D, DRESS syndrome induced by piperacillin-tazobactam: Clin Transl Allergy, 2014; 4(Suppl. 3); P96
14.. Del Pozzo-Magaña BR, Rieder MJ, Garcia-Bournissen F, Lazo-Langner A, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A tertiary care centre retrospective study.: Brit J Clin Pharmacol, 2022; 88(9); 4134-41
15.. Artuković M, Kustelega J, Lugović-Mihić L, DRESS syndrome with mild manifestations as a diagnostic and therapeutic problem: Case report: Acta Clin Croat, 2010; 49(4); 479-84
16.. Truong K, Kelly S, Bayly A, Smith A, Successful mepolizumab treatment for dress-induced refractory eosinophilic myocarditis and concurrent thyroiditis.: BMJ Case Rep., 2021; 14(7); e242240
17.. Alkhateeb H, Said S, Cooper CJ, DRESS syndrome following ciprofloxacin exposure: An unusual association.: Am J Case Rep, 2013; 14; 526-28
18.. Sahnoun R, El Aïdli S, Zaïem A, [DRESS syndrome induced by ciprofloxacine]: Nephrol Ther, 2015; 11(2); 111-13 [in French]
19.. Shiohara T, Mizukawa Y, Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019: Allergol Int, 2019; 68(3); 301-8
20.. Renda F, Landoni G, Bertini Malgarini R, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): A national analysis of data from 10-year post-marketing surveillance: Drug Saf, 2015; 38(12); 1211-18
21.. Yang CY, Chen CH, Deng ST, Allopurinol use and risk of fatal hypersensitivity reactions: A nationwide population-based study in Taiwan: JAMA Intern Med, 2015; 175(9); 1550-57
22.. Chung WH, Chang WC, Stocker SL, Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin: Ann Rheum Dis, 2015; 74(12); 2157-64
23.. Hung SI, Chung WH, Liou LB, HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol: Proc Natl Acad Sci USA, 2005; 102(11); 4134-39 [Erratum in: Proc Natl Acad Sci USA. 2005;102(17):6237]
24.. Cao Z, Wei Z, Zhu Q, HLA-b*58: 01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese.: Pharmacogenomics, 2012; 13(10); 1193-201
25.. Yun J, Marcaida MJ, Eriksson KK, Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58: 01: J Immunol, 2014; 192(7); 2984-93
26.. : LiverTox: Clinical research information on drug-induced liver injury [Internet]. May 4, 2012, Bethesda (MD), National Institute of Diabetes and Digestive and Kidney Diseases Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury.
27.. Chung WH, Chang WC, Stocker SL, Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin.: Ann Rheum Dis, 2015; 74(12); 2157-64
28.. Jamel EG, Ahmed S, DRESS syndrome and chronic renal failure induced by ethambutol: Am J Med Sci, 2019; 358(5); e19
29.. Torroba Sanz B, Mendez Martínez E, Cacho Asenjo E, Aquerreta Gonzalez I, Permanent renal sequelae secondary to Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome induced by quetiapine: Eur J Hosp Pharm, 2021; 28(5); 285-88
30.. Turney R, Skittrall JP, Donovan J, Agranoff D, Drug Reaction, Eosinophilia and Systemic Symptoms (DRESS) syndrome secondary to allopurinol with early lymphadenopathy and symptom relapse.: BMJ Case Rep., 2015; 2015 bcr2015211222
31.. Suzuki Y, Inagi R, Aono T, Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome.: Arch Dermatol, 1998; 134(9); 1108-12
32.. Kano Y, Hiraharas K, Sakuma K, Shiohara T, Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease: Br J Dermatol, 2006; 155(2); 301-6
33.. Hamaguchi Y, Fujimoto M, Enokido Y, Intractable genital ulcers from herpes simplex virus reactivation in drug-induced hypersensitivity syndrome caused by allopurinol: Int J Dermatol, 2010; 49(6); 700-4
34.. Eshki M, Allanore L, Musette P, Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: Arch Dermatol, 2009; 145(1); 67-72
35.. Calle AM, Aguirre N, Ardila JC, Cardona Villa R, DRESS syndrome: A literature review and treatment algorithm: World Allergy Organ J, 2023; 16(3); 100673
36.. Joly P, Janela B, Tetart F, Poor benefit/risk balance of intravenous immunoglobulins in dress: Arch Dermatol, 2012; 148(4); 543
37.. Schmid-Grendelmeier P, Steiger P, Naegeli MC, Benralizumab for severe dress in two COVID-19 patients: J Allergy Clin Immunol, 2021; 9(1); 481-83.e2
Figures
Tables
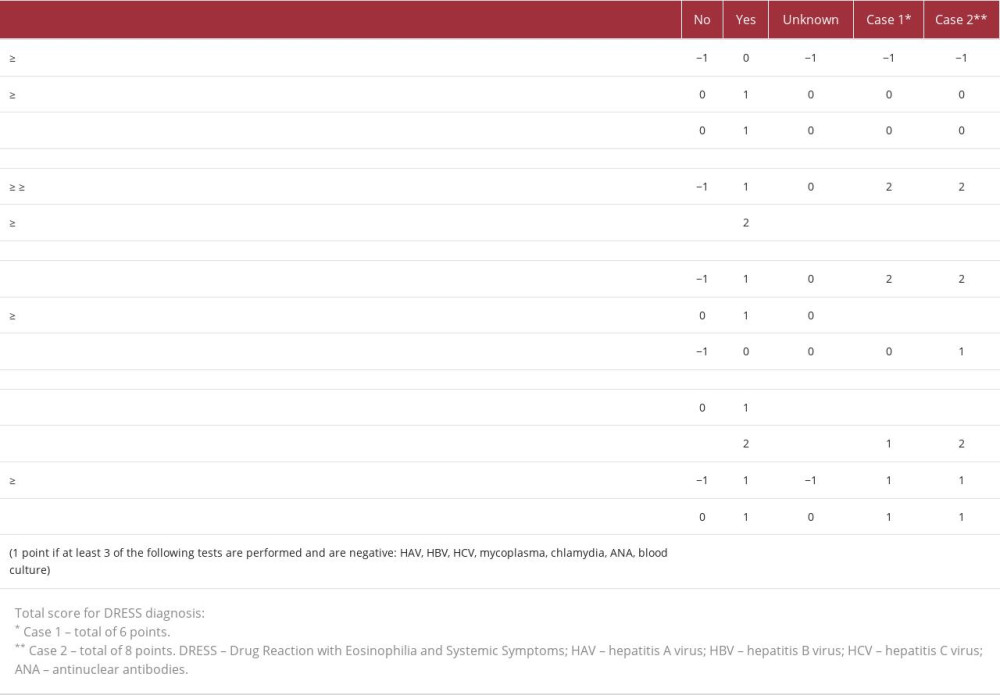 Table 1.. The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) group diagnosis score for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
Table 1.. The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) group diagnosis score for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Table 1.. The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) group diagnosis score for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS).
Table 1.. The Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) group diagnosis score for Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








